ĖâÄŋÄÚČÝ
ĄūĖâÄŋĄŋĘŌÎÂÏÂĢŽCuSO4ĄĪ5H2O(s)ĄĒCuSO4(s)ÓëÆäËŪČÜŌšÖŪžäŨŠŧŊĩÄėĘąäđØÏĩČįÍžĢš

ŌŅÖŠCuSO4ĄĪ5H2O(s)ČÜÓÚËŪĢŽČÜŌšÎÂķČ―ĩĩÍĢŧCuSO4(s)ČÜÓÚËŪĢŽČÜŌšÎÂķČÉýļߥĢ
ÏÂÁÐÓÐđØËĩ·ĻÕýČ·ĩÄĘĮ
A.īÓÁōËáÍČÜŌšÖÐÎöģöCuSO4ĄĪ5H2O(s)ĩÄ·īÓĶėĘąäĄũH>0
B.1mol CuSO4(s)ĩÄŨÜÄÜÁŋīóÓÚ1mol Cu2ĢŦ(aq)Óë1mol SO42Ģ(aq)ĩÄŨÜÄÜÁŋ
C.ĄũH2<ĄũH1
D.ĄũH1Ģ―ĄũH2ĢŦĄũH3
Ąūīð°ļĄŋB
Ąū―âÎöĄŋ
AĄĒCuSO4ĄĪ5H2O(s)ČÜÓÚËŪĢŽČÜŌšÎÂķČ―ĩĩÍĢŽ·īÓĶΊCuSO45H2OĢĻsĢĐ=Cu2+ĢĻaqĢĐ+SO42-ĢĻaqĢĐ+5H2OĢĻlĢĐĄũH2>0ĢŽđĘīÓÁōËáÍČÜŌšÖÐÎöģöCuSO4ĄĪ5H2O(s)ĩÄ·īÓĶėĘąäĄũH<0ĢŽŅĄÏîAīíÎóĢŧ
BĄĒCuSO4(s)ČÜÓÚËŪĢŽČÜŌšÎÂķČÉýļßĢŽ·īÓĶΊCuSO4ĢĻsĢĐ=Cu2+ĢĻaqĢĐ+SO42-ĢĻaqĢĐĄũH3<0ĢŽđĘ1mol CuSO4(s)ĩÄŨÜÄÜÁŋīóÓÚ1mol Cu2ĢŦ(aq)Óë1mol SO42Ģ(aq)ĩÄŨÜÄÜÁŋĢŽŅĄÏîBÕýČ·Ģŧ
CĄĒŌŅÖŠĒŲCuSO45H2OĢĻsĢĐ=Cu2+ĢĻaqĢĐ+SO42-ĢĻaqĢĐ+5H2OĢĻlĢĐĄũH2>0ĢŽĒÚCuSO4ĢĻsĢĐ=Cu2+ĢĻaqĢĐ+SO42-ĢĻaqĢĐĄũH3<0ĢŽļųūÝļĮËđķĻÂÉĢŽÓÉĒŲ-ĒÚĩÃCuSO45H2OĢĻsĢĐ= CuSO4ĢĻsĢĐ+5H2OĢĻlĢĐ ĄũH1=ĄũH2-ĄũH3>0ĢŽÔōĄũH2=ĄũH3+ĄũH1ĢŽđĘĄũH2>ĄũH1ĢŽŅĄÏîCīíÎóĢŧ
DĄĒŌŅÖŠĒŲCuSO45H2OĢĻsĢĐ=Cu2+ĢĻaqĢĐ+SO42-ĢĻaqĢĐ+5H2OĢĻlĢĐĄũH2>0ĢŽĒÚCuSO4ĢĻsĢĐ=Cu2+ĢĻaqĢĐ+SO42-ĢĻaqĢĐĄũH3<0ĢŽļųūÝļĮËđķĻÂÉĢŽÓÉĒŲ-ĒÚĩÃCuSO45H2OĢĻsĢĐ= CuSO4ĢĻsĢĐ+5H2OĢĻlĢĐ ĄũH1=ĄũH2-ĄũH3>0ĢŽŅĄÏîDīíÎóĄĢ
īð°ļŅĄBĄĢ

ĄūĖâÄŋĄŋÄģĘĩŅéÐĄŨéÓÃ0.50 molĄĪL-1 NaOHČÜŌššÍ0.50 molĄĪL-1ÁōËá―øÐÐÖКÍČČĩÄēâķĻĄĢ
ĒņĢŪÅäÖÆ0.50 molĄĪL-1 NaOHČÜŌš
(1)ČôĘĩŅéÖÐīóÔžŌŠĘđÓÃ245 mL NaOHČÜŌš,ÖÁÉŲÐčŌŠģÆÁŋNaOHđĖĖå_____gĄĢ
(2)īÓÏÂąíÖÐŅĄÔņ,ģÆÁŋNaOHđĖĖåËųÐčŌŠĩÄŌĮÆũĘĮ(ĖîŨÖÄļ)________ĄĢ
ÃûģÆ | ÍÐÅĖĖėÆ―(īøíĀÂë) | ÐĄÉÕą | ÛáÛöĮŊ | ēĢÁ§°ô | ŌĐģŨ | ÁŋÍē |
ŌĮÆũ |
|
|
|
|
|
|
ÐōšÅ | a | b | c | d | e | f |
ĒōĢŪēâķĻÏĄÁōËášÍÏĄĮâŅõŧŊÄÆČÜŌšÖКÍČČĩÄĘĩŅéŨ°ÖÃČįÍžËųĘūĄĢ

(1)ČôÉúģÉ1 mol H2OĘą·īÓĶ·ÅģöĩÄČČÁŋΊ57.3 kJ,Ðīģöļ÷īÓĶĩÄČČŧŊŅ§·―ģĖĘ―:__________ĄĢ
(2)ČĄ50 mL NaOHČÜŌššÍ30 mLÁōËá―øÐÐĘĩŅé,ĘĩŅéĘýūÝČįÏÂąíĄĢ
ĒŲĮëĖîÐīÏÂąíÖÐĩÄŋÕ°Ũ:
ĘĩŅéīÎĘý | ÆðĘžÎÂķČt1/Ąæ | ÖÕÖđÎÂķČt2/Ąæ | ÎÂķČēîÆ―ūųÖĩ(t2-t1)/Ąæ | ||
H2SO4 | NaOH | Æ―ūųÖĩ | |||
1 | 26.2 | 26.0 | 26.1 | 30.1 | ______ |
2 | 27.0 | 27.4 | 27.2 | 33.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.8 | |
4 | 26.4 | 26.2 | 26.3 | 30.4 | |
ĒÚÓÃÉÏĘöĘĩŅéĘýÖĩžÆËã―áđûΊ____kJmol-1,Óë57ĢŪ3 kJmol-1ÓÐÆŦēî,ēúÉúÆŦēîĩÄÔŌōŋÉÄÜĘĮ_____(ĖîŨÖÄļ)ĄĢ
aĢŪĘĩŅéŨ°ÖÃąĢÎÂĄĒļôČČЧđûēî
bĢŪÁŋČĄNaOHČÜŌšĩÄĖåŧýĘąŅöĘÓķÁĘý
cĢŪ·ÖķāīΰŅNaOHČÜŌšĩđČëĘĒÓÐÁōËáĩÄÐĄÉÕąÖÐ
dĢŪÓÃÎÂķČžÆēâķĻNaOHČÜŌšÆðĘžÎÂķČšóÖą―ÓēâķĻH2SO4ČÜŌšĩÄÎÂķČ
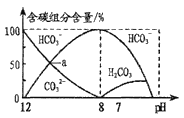
ĄūĖâÄŋĄŋŌŅÖŠēŋ·ÖČõËáĩÄĩįĀëÆ―šâģĢĘýČįÏÂąíĢš
ČõËá | īŨËá | īÎÂČËá | ĖžËá | ŅĮÁōËá |
ĩįĀëÆ―šâģĢĘýĢĻ25ĄæĢĐ | Ka=1.75ĄÁ10Ģ5 | Ka=2.98ĄÁ10Ģ8 | Ka1=4.30ĄÁ10Ģ7 Ka2=5.61ĄÁ10Ģ11 | Ka1=1.54ĄÁ10Ģ2 Ka2=1.02ĄÁ10Ģ7 |
ÏÂÁÐĀëŨÓ·―ģĖĘ―ÕýČ·ĩÄĘĮ
AĢŪÉŲÁŋCO2ÍĻČëNaClOČÜŌšÖÐĢšCO2ĢŦH2OĢŦ2ClOĢĢ―CO![]() ĢŦ2HClO
ĢŦ2HClO
BĢŪÉŲÁŋĩÄSO2ÍĻČëCa(ClO)2ČÜŌšÖÐĢšSO2ĢŦH2OĢŦCa2ĢŦĢŦ2ClOĢĢ―CaSO3ĄýĢŦ2HClO
CĢŪÉŲÁŋĩÄSO2ÍĻČëNa2CO3ČÜŌšÖÐĢšSO2ĢŦH2OĢŦ2 CO![]() Ģ―SO
Ģ―SO![]() ĢŦ2HCO3Ģ
ĢŦ2HCO3Ģ
DĢŪÏāÍŽÅĻķČNaHCO3ČÜŌšÓëNaHSO3ČÜŌšĩČĖåŧýŧėšÏĢšH+ĢŦHCO3ĢĢ―CO2ĄüĢŦH2O