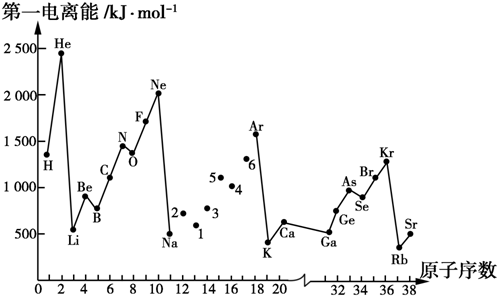
��Ŀ����
5���������ܼ�G��ijҽҩ�м���H��һ�ֺϳ�·�����£����ַ�Ӧ������ȥ����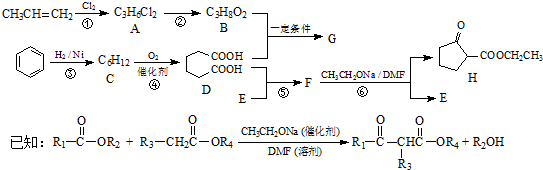
��֪��
��1��B�����ƣ�ϵͳ��������1��2-��������D�ķ���ʽΪC6H10O4��
��2����Ӧ�١���������ȡ����Ӧ���Ǣڢݢޣ�
��3��G�Ľṹ��ʽΪ
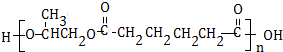 ��
����4��д����Ӧ�ں͢Ļ�ѧ����ʽ����CH3CHClCH2Cl+2NaOH$��_{��}^{ˮ}$CH3CHOHCH2OH+2NaCl����
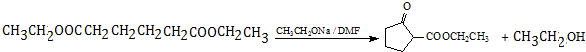 ��
����5��C���ڶ���ͬ���칹�壬д���˴Ź�������ֻ�����ַ��ͬ���칹��Ľṹ��ʽ��
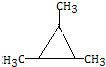 ��
��
���� �����и�����ת����ϵ����ϩ�������ӳɵ�AΪCH3CHClCH2Cl��A����ˮ�⣨ȡ������BΪCH3CHOHCH2OH���������������ӳɷ�Ӧ��CΪ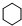 ��C����������Ӧ��D��D��B�����۷�Ӧ��GΪ
��C����������Ӧ��D��D��B�����۷�Ӧ��GΪ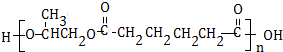 ������������Ϣ��F����ȡ����Ӧ����H��E��D��E����������Ӧ��ȡ����Ӧ����FΪCH3CH2OOCCH2CH2CH2CH2COOCH2CH3������H�Ľṹ����֪EΪCH3CH2OH���ݴ˴��⣮
������������Ϣ��F����ȡ����Ӧ����H��E��D��E����������Ӧ��ȡ����Ӧ����FΪCH3CH2OOCCH2CH2CH2CH2COOCH2CH3������H�Ľṹ����֪EΪCH3CH2OH���ݴ˴��⣮
��� �⣺�����и�����ת����ϵ����ϩ�������ӳɵ�AΪCH3CHClCH2Cl��A����ˮ�⣨ȡ������BΪCH3CHOHCH2OH���������������ӳɷ�Ӧ��CΪ ��C����������Ӧ��D��D��B�����۷�Ӧ��GΪ
��C����������Ӧ��D��D��B�����۷�Ӧ��GΪ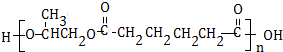 ������������Ϣ��F����ȡ����Ӧ����H��E��D��E����������Ӧ��ȡ����Ӧ����FΪCH3CH2OOCCH2CH2CH2CH2COOCH2CH3������H�Ľṹ����֪EΪCH3CH2OH��
������������Ϣ��F����ȡ����Ӧ����H��E��D��E����������Ӧ��ȡ����Ӧ����FΪCH3CH2OOCCH2CH2CH2CH2COOCH2CH3������H�Ľṹ����֪EΪCH3CH2OH��
��1��BΪCH3CHOHCH2OH��B������Ϊ1��2-������������D�Ľṹ��ʽ��֪��D�ķ���ʽΪC6H10O4��
�ʴ�Ϊ��1��2-��������C6H10O4��
��2����������ķ�����֪����Ӧ�١���������ȡ����Ӧ���Ǣڢݢޣ�
�ʴ�Ϊ���ڢݢޣ�
��3��GΪ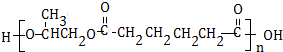 ��
��
�ʴ�Ϊ��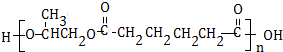 ��
��
��4����Ӧ�ڵĻ�ѧ����ʽΪCH3CHClCH2Cl+2NaOH$��_{��}^{ˮ}$CH3CHOHCH2OH+2NaCl����Ӧ�Ļ�ѧ����ʽΪ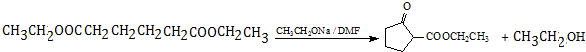 ��
��
�ʴ�Ϊ��CH3CHClCH2Cl+2NaOH$��_{��}^{ˮ}$CH3CHOHCH2OH+2NaCl��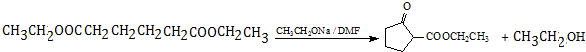 ��
��
��5��CΪ ��C���ڶ���ͬ���칹�壬���к˴Ź�������ֻ�����ַ��ͬ���칹��Ľṹ��ʽΪ
��C���ڶ���ͬ���칹�壬���к˴Ź�������ֻ�����ַ��ͬ���칹��Ľṹ��ʽΪ ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л�����ƶ������ʣ�ע������л���Ľṹ�����ƶϣ���Ҫѧ���Ը������Ϣ�������ã��ϺõĿ���ѧ����ѧ�����������������������ȵ����ͣ��Ѷ��еȣ�

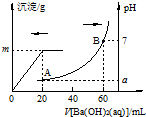 ����ʱ�����������Ļ����Һ20mL������������μ���0.05mol•L-1Ba��OH��2��Һʱ�����ɵ�BaSO4��pH�ı仯��ͼ��ʾ����������Һ���ʱ����ı仯��������˵����ȷ���ǣ�������
����ʱ�����������Ļ����Һ20mL������������μ���0.05mol•L-1Ba��OH��2��Һʱ�����ɵ�BaSO4��pH�ı仯��ͼ��ʾ����������Һ���ʱ����ı仯��������˵����ȷ���ǣ�������| A�� | ͼ��A���Ӧ��Һ��pH��a=1 | |
| B�� | ���ɳ������������m=2.33g | |
| C�� | ԭ���Һ��c��HCl��=0.1mol•L-1 | |
| D�� | ��V[Ba��OH��2��aq��]=30mLʱ���й�����Ũ�ȴ�С��c��Cl-����c��Ba2+����c��H+�� |
| �� | �ױ� | �Ҵ� | ��ϩ | ��ȩ | ������Һ | |
| ���뱥����ˮ���� | �ϲ��Ⱥ�ɫ���²���ɫ | �ϲ��Ⱥ�ɫ���²���ɫ | �����Ա仯 | ��ɫ | ��ɫ | ��ɫ���� |
| ����������������Һ���� | ��Һ�ֲ㣬�ϲ���ɫ | ��ɫ | ��ɫ | ��ɫ | ��ɫ | ��ɫ |
| ���Ƶ�Cu��OH��2 | �ֲ㣬�ϲ���ɫ | �ֲ㣬�ϲ���ɫ | �����Ա仯 | ��ɫ���� | ||
| ����һС�������� | �Ƴ����ײ�������Ӧ | �Ƴ����ײ�������Ӧ | �ų����� |
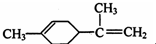
| A�� | ����ϩ��һ�ȴ�����7�� | |
| B�� | ����ϩ�Ͷ�������Ϊͬ���칹�� | |
| C�� | ����ϩ�ķ���������̼ԭ�ӿ�����ͬһ��ƽ���� | |
| D�� | ��һ�������£�����ϩ���Է����ӳɡ�ȡ������������ԭ�ȷ�Ӧ |
| A�� | �μ�KI-������Һ��Ϊ��ɫ | |
| B�� | ����Һ��Cu2+��NH4+��SO42-��Cl- ���Դ������� | |
| C�� | �������ữ��AgNO3��Һ��Ӧ�г������ɲ��ų����� | |
| D�� | �����Һ��ͨ�����Cl2����Ӧ�����ӷ���ʽ��2Fe2++4Br-+3Cl2�T2Fe3++2Br2+6Cl- |
| A�� | $\frac{W��m-n��}{m}$mol | B�� | $\frac{W��m-n+2��}{m}$mol | C�� | $\frac{W��m-n-2��}{m}$mol | D�� | $\frac{m-n-2}{Wm}$mol |
| A�� | �������ں��й��ۼ� | |
| B�� | �����ʵ�Ӳ�ȿ�����ʯ���� | |
| C�� | �����ʳ����³ʹ�̬ | |
| D�� | ��������һ�������¿ɷ����ӳɷ�Ӧ |
| A�� | ��Ȼ���Ƕ�����Դ | B�� | ʯ���Ƕ�����Դ | ||
| C�� | ������һ����Դ | D�� | ˮ����һ����Դ |