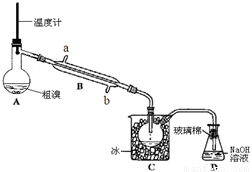
��Ŀ����
���屻��Ϊ����Ԫ�أ�����±��ͨ���������Ƶ��壺Cl2 + 2NaBr = 2NaCl + Br2���÷�Ӧ��������Ԫ��Ϊ
__________��дԪ�ط��ţ�����������Ӧ�Ƶ�16g Br2����ת�Ƶĵ�����Ŀ��_________����
���밴Ҫ����д��ѧ����ʽ�����ӷ���ʽ
��1��С�����û�ѧʵ��֤���˾����ڿ����е�Ư���ѱ��ʣ����û�ѧ����ʽ��ʾƯ�۱��ʵ�ԭ��
__________________��
��2��FeSO4��Һ��ϡH2SO4�ữ������һ��ʱ������Ի�ɫ��д���仯���̵����ӷ���ʽ__________________��Ȼ�������еμ�KI-������Һ����ɫ��д���仯���̵����ӷ���ʽ___________________��
__________��дԪ�ط��ţ�����������Ӧ�Ƶ�16g Br2����ת�Ƶĵ�����Ŀ��_________����
���밴Ҫ����д��ѧ����ʽ�����ӷ���ʽ
��1��С�����û�ѧʵ��֤���˾����ڿ����е�Ư���ѱ��ʣ����û�ѧ����ʽ��ʾƯ�۱��ʵ�ԭ��
__________________��
��2��FeSO4��Һ��ϡH2SO4�ữ������һ��ʱ������Ի�ɫ��д���仯���̵����ӷ���ʽ__________________��Ȼ�������еμ�KI-������Һ����ɫ��д���仯���̵����ӷ���ʽ___________________��
�� Br��0.2NA��1.204��1023
��1��Ca(ClO)2+CO2+H2O��CaCO3+2HClO��2HClO 2HCl+O2��
2HCl+O2��
��2��4Fe2++ 4H+ + O2 ��4Fe3++2H2O��2Fe3++2I-��2Fe2++I2

��ϰ��ϵ�д�
�����Ŀ