��Ŀ����
ijУ��ѧʵ����ȤС��Ϊ��̽����ʵ�����Ʊ�Cl2�Ĺ�������ˮ������HCl�ӷ�������ͬʱ֤��������ijЩ���ʣ���ͬѧ���������ͼ��ʾ��ʵ��װ�ã�֧���õ�����̨ʡ�ԣ�����Ҫ��ش����⡣
��1�����з�����,���Ƶ���������ȷ����� ��
��MnO2��Ũ�����Ϲ���
��KMnO4��Ũ������ �������ƺ�Ũ������ ��K2Cr2O7��Ũ������
| A���٢ڢ� | B���٢ڢ� | C���٢� | D��ȫ������ |
��3����װ��B�������� �������� ��
��װ��C��D���ֵIJ�ͬ����˵���������� ��
��װ��E��������
Ŀǰ�ҹ�����ˮ������Ҫ���ȡ���ͨ��������Ư�ۻ�Ư����
��1�� Ư�ۻ�Ư�����ڷ����ڿ����л����ʧЧ���仯ѧ����ʽ��__________________��
��2��Ŀǰ�ҹ��㷺���þ����������ϡ�͵�������ͨ�����й�����������(NaClO2)������
�Ƶ�ClO2����һ��Ӧ�Ļ�ѧ����ʽ�ǣ�_________________________________________��
��1��D ��2��Ũ�������ŷ�Ӧ�Ľ���Ũ�����ͣ����ձ�Ϊϡ���ᣬ��Ӧ���ٲ�������
��3����֤����ˮ�������� ��ɫ�������ɫ
��������Ư���ԣ�������ˮ��Ӧ�����Ĵ�������Ư����
����������
��1��Ca(ClO)2+CO2+H2O=CaCO3��+2HClO
��2��Cl2+2NaClO2=2ClO2+2NaCl
���������������1��MnO2��KMnO4��NaClO3��K2Cr2O7������ǿ�����ԣ���Ũ���ᷴӦ��������Cl2����D����ȷ��
��2��Ũ�������ŷ�Ӧ�Ľ���Ũ�����ͣ����ձ�Ϊϡ���ᣬ��Ӧ���ٲ���������HClΪ0��2mol���������ɵ�Cl2С��0��05mol��С��1��12L��
��3����װ��B��ʢ����ˮ����ͭ�����������Ϊ֤��ˮ�����Ĵ��ڣ�����Ϊ����ɫ�������ɫ��
��װ��D��E���������ڲ����Ƿ�������ʪ��IJ����У�������ˮ��Ӧ���ɴ����ᣬ���������װ��˵���ˡ�������Ư���ԣ���������Ư���ԡ���
��Ҫ������HCl�ӷ���������Ҫ��ȥCl2������װ��E�������ǣ�����������
Ư�۳��ڷ����ڿ����У�Ca(ClO)2��CO2��H2O��Ӧ����HClO��HClO�ֽ�ʹƯ��ʧЧ��
Cl2��NaClO2�е�+3��Cl����ΪClO2����ƽ�ɵû�ѧ����ʽ��
���㣺���⿼����������ȡ�����ʡ���ѧ����ʽ����д��

ij��ȤС����Ʋ�����������ʵ����̽��Cl2��Ư�۵��Ʊ����й����ʡ�
��1��ʵ������������װ���Ʊ����﴿�����������밴������������������ķ��������������ӣ�
H�������������������������������������������������������ƿ���е��Լ�Ϊ��������������������������
��2��д����ҵ����������ʯ������ȡƯ�۵Ļ�ѧ��Ӧ����ʽ��
������������������������������������������������������������������������������������������
ijѧ���������ʵ���һ��̽��SO2��Ư�۾��ķ�Ӧ��
| ���� | ���� |
| ȡ4 gƯ�۾����壬����100 mLˮ | ���ֹ����ܽ⣬��Һ������ɫ |
| ���ˣ���Ư�۾���Һ��pH | pH��ֽ�ȱ�����ԼΪ12��������ɫ |
 A | ��.Һ���Ϸ�������״�� ��.�Ժ��ֻ��ǣ���Һ��Ϊ����ɫ ��.�Ժ���������ɫ����������ɫ��ȥ |
��3��pH��ֽ��ɫ�ı仯˵��Ư�۾���Һ���е�������������������������������������������
��4����ͬѧ�Ʋ�����i����״��������СҺ���γɣ���������ʵ����Խ�һ����֤��
a����ʪ��ĵ⻯�ص�����ֽ������״��ޱ仯��
b���Ѽ���״����ữ��AgNO3��Һ���飬������ɫ������
ʵ��a��Ŀ��������������������������������������������������������������������������
��5����������Һ��Ϊ����ɫ�Ŀ���ԭ������Һ���Ե���ǿ��Ư�۾���ijЩ�ɷ�֮�䷢����Ӧ�������ʵ�鷽������һ��ȷ�����ֿ����ԣ�����Ϊ��������������������������������������������
������������������������������������������������������������������������������������
��6���û�ѧ����ʽ���������л���ɫ��ȥ��ԭ����������������������ɫ����Һ���Ƿ���
 �ķ����ǣ��� ����������������������������������������
�ķ����ǣ��� ���������������������������������������� ij��ȤС���������ͼʵ��װ�ý���ʵ�顣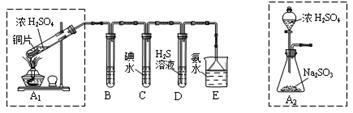
��̽��������Ⱦ��SO2������
��1��Ϊ��ʵ����ɫ������Ŀ�꣬�ܷ�����ͼA2����A1װ�� ����ܡ�����
��2��B��C��D�ֱ����ڼ���SO2��Ư���ԡ���ԭ�Ժ������ԣ���B����ʢ�Լ�Ϊ ��C�з�Ӧ�����ӷ���ʽΪ ��D�з�Ӧ�Ļ�ѧ����ʽΪ ��
��̽��ͭƬ��ŨH2SO4��Ӧ�IJ���
ʵ�������������ͭƬ���渽�ź�ɫ���塣�������ϵ�֪���˺�ɫ������ܺ���CuO��CuS��Cu2S��������CuS��Cu2S��������ϡ���ᣬ�ڿ��������ն�ת��ΪCu2O��SO2����С��ͬѧ�ռ�һ������ɫ���壬������ʵ�鷽��̽����ɷ֣�
��3��������м�������ϴ�Ӹɾ���ʵ�鷽����_____________________________��
��4����ɫ����ijɷ���________________��
�������
�ð�ˮ����β���е�SO2��������Һ���п��ܺ���OH-��SO32-��SO42-��HSO3-�������ӡ�
��5����ˮ���չ���SO2�ķ�Ӧ�����ӷ���ʽΪ ��
��6����֪����������һ��������ˮ��SO2Ҳ������ˮ�������������Լ�Ϊ��С�ձ����Թܡ�����������ͷ�ιܡ�����װ�ú���ֽ��2 mol/L���ᡢ2 mol/LHNO3��1 mol/LBaCl2��Һ��l mol/LBa(OH)2��Һ��Ʒ����Һ������ˮ�������ʵ��֤��������Һ���д���SO32-��HSO3-������±���ʵ�������Ԥ������ͽ��ۣ�
| ʵ����� | Ԥ����������� |
| ����1��ȡ����������Һ����С�ձ��У��ý�ͷ�ι�ȡl mol/L BaCl2��Һ��С�ձ��μ�ֱ�������� | �����ְ�ɫ���ǣ� ������Һ���д���SO32-�� SO42-�� |
| ����2����С�ձ��е���Һ���ˡ�ϴ�ӣ���������ˮ�Ѹ�����ֽ�ϵĹ��������һС�ձ��У�����µĹ��� �� | �� ������Һ���д��� SO32-�� |
| ����3�� �� | �� ������Һ���д��� HSO3-�� |
ijѧУ��ȤС���������װ�ý���ʵ��̽����a��bΪ���ɼУ����ȼ��̶�װ������ȥ����
��1����֤̼����ǽ����Ե����ǿ��
������������ ����ҩƷ��a�ر�b��Ȼ�����Ũ���ᣬ���ȣ�
��װ��A�е��Լ��� ��
����˵��̼�ķǽ����Աȹ�ǿ��ʵ�������� ��
��2����֤SO2�������ԡ���ԭ�Ժ������������ͨ��
�ٴ�b���ر�a������֤SO2���������ԵĻ�ѧ����ʽ�� ��
����������SO2ͨ��NaOH��Һ�У������ӷ���ʽ�� ��
��BaCl2��Һ������������ֳ����ݣ��ֱ�μ�������Һ���������ij����Ļ�ѧʽ�����±���Ӧλ�á�
| �μӵ���Һ | ��ˮ | ��ˮ |
| �����Ļ�ѧʽ | | |
д������SO2��ʾ��ԭ�Բ����ɳ��������ӷ���ʽ ��






