��Ŀ����
�������һ�����ᣬ��������ʴ��������֪25 ��ʱ��
��HF(aq)+OH-(aq)===F-(aq)+H2O(l)��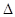 H=��67.7 kJ��mol-1
H=��67.7 kJ��mol-1
��H+(aq)+OH-(aq)===H2O(l)��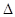 H=��57.3 kJ��mol-1
H=��57.3 kJ��mol-1
��20 mL 0.1 mol��L-1������м���V mL 0.1 mol��L-1 NaOH��Һ�������й�˵����ȷ���ǣ�����
��HF(aq)+OH-(aq)===F-(aq)+H2O(l)��
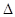 H=��67.7 kJ��mol-1
H=��67.7 kJ��mol-1��H+(aq)+OH-(aq)===H2O(l)��
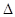 H=��57.3 kJ��mol-1
H=��57.3 kJ��mol-1��20 mL 0.1 mol��L-1������м���V mL 0.1 mol��L-1 NaOH��Һ�������й�˵����ȷ���ǣ�����
A�������ĵ��뷽��ʽ����ЧӦ�ɱ�ʾΪ��HF(aq) F-(aq)+H+(aq)�� F-(aq)+H+(aq)��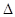 H=��10.4 kJ��mol-1 H=��10.4 kJ��mol-1 |
| B����V=20ʱ����Һ�У�c(OH-)=c(HF)+c(H+) |
| C����V=20ʱ����Һ�У�c(F-)��c(Na+)=0.1 mol��L-1 |
| D����V��0ʱ����Һ��һ�����ڣ�c(Na+)��c(F-)��c(OH-)��c(H+) |
B
���������1ʽ��2ʽ���ɵõ����������ķ���ʽ��
 H=��10.4 kJ��mol-1��A��
H=��10.4 kJ��mol-1��A��B����V=20ʱ����������������ᣬ���Ի�Ϻ���Һ�ʼ��ԣ����������غ�ɵ�c(OH-)=c(HF)+c(H+)���ʶԡ�
C����V=20ʱ�����ڻ��Һ������ӱ����������ʵ�����Ũ�Ƚ���һ�룬c(F-)��c(Na+)=0.05mol?L-1�ʴ�
D����V��0ʱ����V=1ʱ�����������ӵ����ʵ���ԶԶС�ڷ����ӣ�������Һ��һ��������ڣ�c(Na+)��c(F-)��c(OH-)��c(H+)���ʴ�
������������Ҫ�������������ĵ���ͷ����ӵ�ˮ�⣬���Һ�и�����Ũ�ȴ�С�ıȽϣ����ü��跨���Լ��ٵ��ҵ���ȷ�𰸡�

��ϰ��ϵ�д�
 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д� ����������ϵ�д�
����������ϵ�д�
�����Ŀ
 2SO3(g) ��H="-Q" kJ��mol-1(Q��0)�����SO2��ת����Ϊ90%��
2SO3(g) ��H="-Q" kJ��mol-1(Q��0)�����SO2��ת����Ϊ90%�� ��
�� ��Ӧ�����������仯������ͼ������ͼ���ж�������������ȷ����
��Ӧ�����������仯������ͼ������ͼ���ж�������������ȷ����
 2NH3(g)��H=+92kJ/mol
2NH3(g)��H=+92kJ/mol �����Ǽ������ʱ�������仯����
�����Ǽ������ʱ�������仯���� N2(g)+3H2(g) ��H=+92kJ/mol
N2(g)+3H2(g) ��H=+92kJ/mol

 �÷�Ӧ��ƽ�ⳣ������ʽΪ ��
�÷�Ӧ��ƽ�ⳣ������ʽΪ ��
 N2(g)+
N2(g)+ H2(g)��NH3(1)����H��(b+c-a)kJ��mol��1
H2(g)��NH3(1)����H��(b+c-a)kJ��mol��1