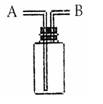
��Ŀ����
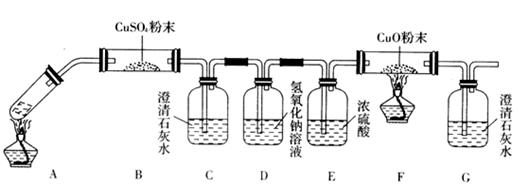
��20�֣�ij����С������H2��ԭCuO��ĩ�ⶨͭԪ�ص����ԭ����������ͼ�Dzⶨװ�õ�ʾ��ͼ��A�е��Լ������ᣬC���Լ���ϡ����������Һ��

��ش��������⡣
��1��������װ����Լ���B ��D ��
��2��װ��C�������� ��
��3�����Ӻ�װ�ú�Ӧ���� ��
��4�����ټ��ȷ�Ӧ��E���͡��ڴ�Aƿ��εμ�Һ�塱����������Ӧ���Ƚ��е��� ������ţ�����������֮�仹Ӧ���еIJ����� ��
��5����Ӧ������G���ݳ��������� ���䴦�������� ��
��6����ʵ���в�����������ݣ�
�ٿ�E�ܵ�����a����E�ܺ�CuO��������b��
�۷�Ӧ��E�ܺ�Cu�۵�������c����ȴ�����³�������
�ܷ�ӦǰF�ܼ���ʢ���������d���ݷ�Ӧ��F�ܼ���ʢ���������e��
���������ݿ����г�����Cu�����ԭ��������������ͬ����ʽ����Cu�⣬�����漰��Ԫ�ص����ԭ��������Ϊ��֪����
����ʽ1��Ar(Cu)�� ������ʽ2��Ar(Cu )�� ��

��ش��������⡣
��1��������װ����Լ���B ��D ��
��2��װ��C�������� ��
��3�����Ӻ�װ�ú�Ӧ���� ��
��4�����ټ��ȷ�Ӧ��E���͡��ڴ�Aƿ��εμ�Һ�塱����������Ӧ���Ƚ��е��� ������ţ�����������֮�仹Ӧ���еIJ����� ��
��5����Ӧ������G���ݳ��������� ���䴦�������� ��
��6����ʵ���в�����������ݣ�
�ٿ�E�ܵ�����a����E�ܺ�CuO��������b��
�۷�Ӧ��E�ܺ�Cu�۵�������c����ȴ�����³�������
�ܷ�ӦǰF�ܼ���ʢ���������d���ݷ�Ӧ��F�ܼ���ʢ���������e��
���������ݿ����г�����Cu�����ԭ��������������ͬ����ʽ����Cu�⣬�����漰��Ԫ�ص����ԭ��������Ϊ��֪����
����ʽ1��Ar(Cu)�� ������ʽ2��Ar(Cu )�� ��
��1��п����1�֣���Ũ�����2����ȥ�����л��е��Ȼ�������
��3����������ԣ�4���ڣ�����H2�Ĵ��ȣ�5��������1�֣�����G�ܳ��ڴ���ȼ��
��6��
 ����3�֣�
����3�֣�
��������վ�Ϊ2�֣�
��3����������ԣ�4���ڣ�����H2�Ĵ��ȣ�5��������1�֣�����G�ܳ��ڴ���ȼ��
��6��

 ����3�֣�
����3�֣���������վ�Ϊ2�֣�
��

��ϰ��ϵ�д�
 ״Ԫ����ϵ�д�
״Ԫ����ϵ�д� ͬ������ϵ�д�
ͬ������ϵ�д�
�����Ŀ
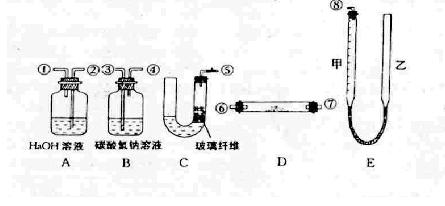
 OC�DCOOH���ɼ�дΪH2C2O4)�׳Ʋ��ᣬ������ˮ�����ڶ�Ԫ��ǿ��(Ϊ�������)��������ǿ��̼�ᣬ���۵�Ϊ101.5�棬��157��������ijУ�о���ѧϰС��Ϊ̽������IJ��ֻ�ѧ���ʣ�����������ʵ�飺
OC�DCOOH���ɼ�дΪH2C2O4)�׳Ʋ��ᣬ������ˮ�����ڶ�Ԫ��ǿ��(Ϊ�������)��������ǿ��̼�ᣬ���۵�Ϊ101.5�棬��157��������ijУ�о���ѧϰС��Ϊ̽������IJ��ֻ�ѧ���ʣ�����������ʵ�飺 HCO3��Һ���Թ��м��������Ҷ�����Һ���۲쵽����ɫ���ݲ������÷�Ӧ�����ӷ���ʽΪ_______
HCO3��Һ���Թ��м��������Ҷ�����Һ���۲쵽����ɫ���ݲ������÷�Ӧ�����ӷ���ʽΪ_______ __________________________________��
__________________________________��

 �� ����Һ���¶ȣ�NO2 21�棬 NO -152��
�� ����Һ���¶ȣ�NO2 21�棬 NO -152��