��Ŀ����
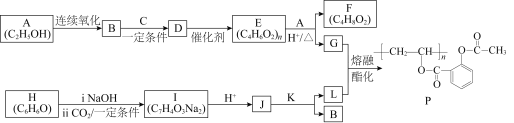
����Ŀ����˾ƥ�֣�������L����������֪�Ľ�����ʹҩ�һ�ֳ�Ч�����Ͱ�˾ƥ�֣�������P���ĺϳ�·������ͼ��ʾ��
��֪����HC��CH+RCOOH![]()
![]()
��RCOOR��+R��OH![]() RCOOR��+R��OH��R��R����R������������
RCOOR��+R��OH��R��R����R������������
��ش�
(1)A�еĹ�������____________________��
(2)C�Ľṹ��ʽ��____________________��
(3)D��E�ķ�Ӧ������____________________��
(4)E��G�Ļ�ѧ����ʽ��______________________________________��
(5)��֪��H�Ƿ����廯�����һ��������2B �� K + H2O��K�ĺ˴Ź�������ֻ��һ��塣J��L�Ļ�ѧ����ʽ��____________________��
(6)L�����ڿɽϿ�ת��Ϊ����ҩЧ��J����������P��L��ȣ��������ܻ��������ͷ�J��
�� ѪҺ��JŨ�ȹ�����ʹ���ж����ɾ�����עNaHCO3��Һ�ⶾ�����û�ѧ����ʽ����NaHCO3�����ã�______________________________________________________________��
�� ����˵����ȷ����______������ĸ����
a��P�е����������ڿɻ���ˮ�⣬���ͷų�J
b��P�����ڵ�ˮ�������û�и߷��ӻ�����
c����С����ҩ�����뵽�߷����п���ʵ��ҩ��Ļ�����
���𰸡��ǻ� HC��CH �Ӿ۷�Ӧ 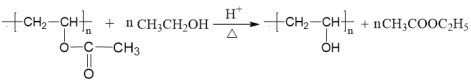

![]() ac
ac
��������
A��![]() ���Ҵ��������������ᣬ��B��
���Ҵ��������������ᣬ��B��![]() ��������Ϣ�٣��������ͼ��C��
��������Ϣ�٣��������ͼ��C��![]() ��D��
��D��![]() ��D��E�ǼӾ۷�Ӧ��E��
��D��E�ǼӾ۷�Ӧ��E�� ��������Ϣ�ڿ���֪FΪ
��������Ϣ�ڿ���֪FΪ![]() ��G��
��G��![]() �����ݷ���ʽH��
�����ݷ���ʽH��![]() ����������ͼ���P�Ľṹ����֪IΪ
����������ͼ���P�Ľṹ����֪IΪ ��J��
��J��![]() �����ݣ�5��С�⣬������֪K����������
�����ݣ�5��С�⣬������֪K����������![]() ������P����֪LΪ
������P����֪LΪ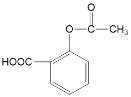 ��
��
��1��A���Ҵ����Ҵ��Ĺ��������ǻ���
��Ϊ���ǻ���
��2������Ϣ�٣��������ͼ�������Ƴ�C��![]() ��
��
�ʴ�Ϊ��![]() ��
��
��3��D��![]() �� E��
�� E�� ��D��E��˫�������˼Ӿ۷�Ӧ��
��D��E��˫�������˼Ӿ۷�Ӧ��
�ʴ�Ϊ���Ӿ۷�Ӧ��
��4��E�� ��A���Ҵ���������Ϣ�ڣ��÷�ӦΪȡ����Ӧ��
��A���Ҵ���������Ϣ�ڣ��÷�ӦΪȡ����Ӧ��
���� ��
��
��5��������Ϣ����֪J��![]() ��K����������
��K���������� ����
����
����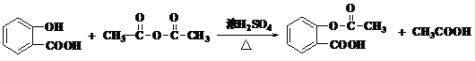 ��
��
��6������������Դ���̼�ᣬ�ӵ�����С��̼�ᣬ �ʷ��ǻ���̼�����Ʋ���Ӧ���Ȼ���̼�����Ʒ�Ӧ���ɶ�����̼��
�ʴ�Ϊ��![]() ��
��
��a.P������ȫˮ�����![]() ��
��![]() �Լ����ᣬa��ȷ��
�Լ����ᣬa��ȷ��
b. P������ȫˮ�����![]() ��������Ϊ�߷��ӣ�b����
��������Ϊ�߷��ӣ�b����
c. ������Ŀ��Ϣ����С����ҩ�����뵽�߷����п���ʵ��ҩ��Ļ����ܣ�c��ȷ��
��ѡac��

����Ŀ��AlN���Ͳ���Ӧ��ǰ���㷺�����Ʊ��������о���Ϊ�ȵ㡣
����������£�
���� | �۵�/�� | �е�/�� | ��N2��Ӧ�¶�/�� | ��Ӧ������ֽ��¶�/�� |
Al | 660 | 2467 | ��800 | AlN����2000 ����1400������ AlCl3������181������ |
Mg | 649 | 1090 | ��300 | Mg3N2����800 |
(1)AlN���Ʊ���
�� ��ѧ�����������
��.һ���¶��£���AlCl3�����NH3Ϊԭ���Ʊ�AlN����Ӧ�Ļ�ѧ����ʽ��____________________��
��.������Ӧ���˵��¶ȷ�Χ��______�棨����ĸ����
a.75~100 b.600~1100 c.2000~2300
�� ����ֱ�ӵ�������
Al��N2��ֱ�ӻ���ΪAlN���壬AlN�ܽ�Al��������Ӧ���Լ������С������¶ȣ���Al���о��Ȳ�������Mg�ۣ���ʹAl����ȫ��ת��ΪAlN���塣�ù��̷����ķ�Ӧ�У�__________________��_________��2Al + N2 ![]() 2AlN��
2AlN��
��̼�Ȼ�ԭ����
��Al2O3��C��ʯī����N2Ϊԭ�ϣ��ڸ������Ʊ�AlN��
��֪����. 2Al2O3(s) 4Al(g) + 3O2(g) H 1 =��3351 kJ��mol-1
��. 2C(ʯī��s) + O2(g) = 2CO(g) H 2 =��221 kJ��mol-1
��. 2Al(g) + N2(g) = 2AlN(s) H 3 =��318 kJ��mol-1
����ƽ���ƶ�ԭ��������Ӧ���Է�Ӧ���Ŀ���Ӱ�죺______________________________________��
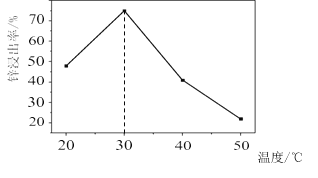
(2)AlN�����ʡ�AlN��ĩ�ɷ���ˮ�⡣��ͬ�����£���ͬ������AlN��ĩˮ��ʱ��ҺpH�ı仯��ͼ��ʾ��
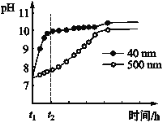
�� AlN��ĩˮ��Ļ�ѧ����ʽ��____________________________________��
�� ����t1-t2ʱ�����������߲���Ŀ���ԭ��_______________________________��
(3)AlN������⡣��a g AlN��Ʒ�м�������ŨNaOH��Һ��Ȼ��ͨ��ˮ������NH3ȫ����������NH3�ù�����v1 mL c1 mol��L-1 H2SO4��Һ������ȫ��ʣ���H2SO4��v2 mL c2 mol��L-1 NaOH��Һǡ���кͣ�����Ʒ��AlN������������________________________________��
����Ŀ��CoCl2�����ڵ�ƣ���һ��������Խ�ĵ��ǰ�����ϣ��ɺ��ܿ�(CoԪ����Ҫ��Co2O3��CoO���ڣ�������Fe��Si��Cu��Zn��Mn��Ni��Mg��CaԪ��)��ȡ�Ȼ��ܾ����һ�ֹ����������£�
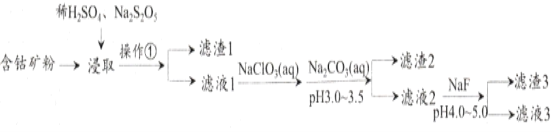
��֪���ٽ���������Na2S2O5������ʳƷ����������CaF2��MgF2������ˮ��
��CoCl2��6H2O�۵�86�棬������ˮ�����ѵȣ��������ȶ�����������110��120��ʱ��ʧȥ�ᾧˮ����ж�����ˮ�Ȼ��ܡ�
�۲��ֽ��������γ����������pH���±���
Co3+ | Fe3+ | Cu2+ | Co2+ | Fe2+ | Zn2+ | Mn2+ | Mg2+ | |
��ʼ����pH | 0.3 | 2.7 | 5.5 | 7.2 | 7.6 | 7.6 | 8.3 | 9.6 |
��ȫ����pH | 1.1 | 3.2 | 6.6 | 9.2 | 9.6 | 9.2 | 9.3 | 11.1 |
�ش��������⣺
��1�������ٵ�����Ϊ_________��NaClO3���������ԣ�������Ϊ__________________��
��2����ȡ�м���Na2S2O5��������___________________________��
��3����Һ1�м���NaClO3/span>��������_______________________________________����ص����ӷ���ʽΪ__________________________________________��
��4������Na2CO3��Һ��������2����Ҫ���ӷ���ʽΪ___________________________��
��5������3��Ҫ�ɷ�Ϊ________________________(д��ѧʽ)��
��Һ3���������ȡ�뷴��ȡ�Ʊ�CoCl2����
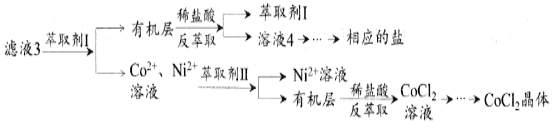
��6����Һ3�м�����ȡ��I��Ȼ����ϡ���ᷴ��ȡ��Ŀ����_______________________��
��7���Ʊ�����CoCl2��6H2O�����ڼ�ѹ�����º�ɵ�ԭ����_________________________________��
����Ŀ���к��ȵIJⶨ�Ǹ�����Ҫ�Ķ���ʵ�顣ȡ0.55mol/L��NaOH��Һ50mL��0.25mol/L������50mL������ͼ��ʾ��װ���н����к��ȵIJⶨʵ�飬�ش��������⣺

��1������ͼʵ��װ�ÿ���������ȱ�ٵ�һ�ֲ�����Ʒ��__________������֮�⣬װ���е�һ�����Դ�����___________________________________��
��2��Ϊ��֤��ʵ��ɹ���ͬѧ��ȡ�������ʩ����ͼ����ֽ������������_________��
��3��������60mL 0.25mol��L-1 H2SO4��50mL 0.55mol��L-1 NaOH��Һ���з�Ӧ������ʵ����ȣ����ų�������_______��������������������������ʵ���������ȷ���������к���__________����������������������
��4������NaOH��Һ����ȷ�����ǣ�________����������ѡ����
A���ز������������� B���������������� C��һ��Ѹ�ٵ���
��5��ʵ���������±���������д�±��еĿհף�
�¶� ʵ����� | ��ʼ�¶�t1�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ (t2��t1)/�� | ||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 29.5 | ___________ |
2 | 27.0 | 27.4 | 27.2 | 32.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.2 | |
4 | 26.4 | 26.2 | 26.3 | ||
�ڽ�����Ϊ0.55 mol/L NaOH��Һ��0.25 mol/L������Һ���ܶȶ���1 g/cm3���кͺ�������Һ�ı�����c��4.18 J/(g����)�����к�����H��_________��(��ʾ����H=��![]() ,����һλС��)��
,����һλС��)��
������ʵ����ֵ�����57.3 kJ/mol��ƫ�����ƫ���ԭ�������(����ĸ)________��
a��ʵ��װ�ñ��¡�����Ч����
b�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
��6������ú�0.5mol Ba(OH)2��ϡ��Һ������ϡ������Һ��Ӧ����Ӧ�ų�����____57.3 kJ����������������С������������������