��Ŀ����
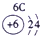
��16�֣��±�ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã�����Ӧ��ѧ����ش��������⣺
| �� ���� | IA | | 0 | |||||
| 1 | �� | IIA | IIIA | IVA | VA | VIA | VIIA | |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | �� | �� | | �� | �� | |
д������ԭ�Ӱ뾶��С��ԭ���γ�������Ϊƽ��ṹ�Ļ�����ĵ���ʽ_____��
��2���ܢݢ��γɵļ����Ӱ뾶�ɴ�С��˳��Ϊ_______________�������ӷ��ţ����ۢߢ������������Ӧˮ�����������ǿ������˳��Ϊ_____________________�����ѧʽ��
��3����ҵ���âڵ��ʴ��������ߵ��ʣ���Ӧ�Ļ�ѧ����ʽΪ��________________________________
���γ�������ľ�������Ϊ__________,����һ����Ҫ��;Ϊ ��
��4����ЩԪ���γɵ��������У�������ˮ����������ǿ��������ǿ�Ӧ����___________���ѧʽ����д������ݵ�����������Ӧˮ�������Ӧ�����ӷ���ʽ_________________________________��
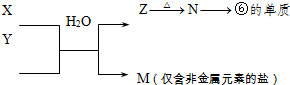
��5��X��Y�ɢ٢ڢ��е����ֻ�����Ԫ����ɡ�X����Һ����С�մ�Ӧ����Y����X��������ϵ������ķ��ӣ�����Է�������Ϊ46����X������Ϊ ��д��X��Һ��С�մ�Ӧ�Ļ�ѧ����ʽΪ____________��
(��16��)��1�� ��1�֣�
��1�֣� ��1�֣�
��1�֣�
��2��S2->O2->Na +��2�֣� HClO4>HNO3>H2SiO3 ��2�֣�
��3��SiO2 +2C Si+ 2CO����2�֣�ԭ�Ӿ��� ���ά����1�֣�
Si+ 2CO����2�֣�ԭ�Ӿ��� ���ά����1�֣�
��4��Al2O3��1�֣� Al2O3 + 2 OH�� =" 2" AlO2�� + H2O��2�֣�
��5������ (1��) HCOOH + NaHCO3 = HCOONa + CO2�� + H2O��2�֣�
����

��ϰ��ϵ�д�
 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д�
�����Ŀ








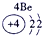
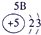
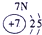
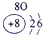
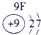
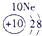
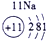





 ��ʾ����
��ʾ����