��Ŀ����
����Ŀ�������ҹ���ҵ��ˮƽ�IJ��Ϸ�չ�����ˮ��������Ⱦ�����Ϊ��Ҫ���⡣
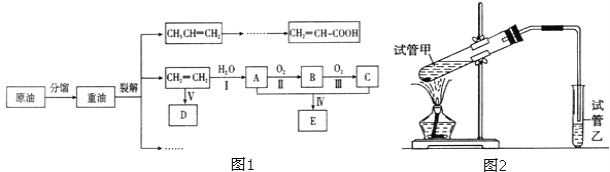
(1)��ҵβ���к��д����ĵ������NH3����ԭ��������(SCR)������ĿǰӦ����㷺���������������ѳ���������Ӧԭ����ͼ��ʾ��
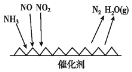
����ͼ��֪SCR�����е�������Ϊ____��
����Fe����������ʱ���ڰ�������������£���NO2��NO�����ʵ���֮��Ϊ1��1ʱ��д���÷�Ӧ�Ļ�ѧ����ʽ��_______��
(2)ClO2������һ�ֳ��õ������������ڱ��㷺��������ˮ����������ˮ����ClO2�������ˮ�У�Ҫ��ClO2��Ũ����0.1~0.8 mg/L�����������Լ��ˮ��ClO2��Ũ�ȣ��������£�
��.ȡһ�������ˮ������������������Һ�������ԣ�Ȼ�����һ�����ĵ⻯�أ������������Һ����Һ����![]()
��.����һ������Na2S2O3��Һ(��֪��2S2O32-+I2=S4O62-+2I-)
��.���������ˮ��pH��1.3��
��֪��ClO2�����������»�ԭ����ΪClO2-�������������»�ԭ����ΪCl-����ش��������⣺
��ȷ����������ȫ��Ӧ������Ϊ________��
���ڲ���������У���Һ�ֳ���ɫ����Ӧ�����ӷ���ʽΪ______��
����ˮ�������Ϊ1.0 L���ڲ�����ʱ������1.0��10-3 mol/L��Na2S2O3��Һ10 mL����ˮ����ClO2��Ũ����____mg/L��
���𰸡�NO��NO2 2NH3+NO+NO2![]() 2N2+3H2O ��ɫ��ʧ��������ڲ���ɫ ClO2-+4I-+4H+=Cl-+2I2+2H2O 0.675
2N2+3H2O ��ɫ��ʧ��������ڲ���ɫ ClO2-+4I-+4H+=Cl-+2I2+2H2O 0.675
��������
(1)�ٵõ��ӣ����ϼ۽��͵ķ�Ӧ������������
�ڸ��ݻ��ϼ�����������ȡ�ԭ���غ�����ƽ��
(2)�ټ���һ���� Na2S2O3 ��Һ��������Ӧ2S2O32-+I2=S4O62-+2I-��ʹI2��ԭΪI-��
����ͼʾ������pH��1��3ʱ��ClO2-��I-�������ɵ�I2����۽���ٴγ�����ɫ�����ݻ��ϼ�����������ȡ�ԭ���غ�͵���غ�����ƽ��
�۸��ݹ�ϵʽClO2��I-��S2O32-�ȼ����ClO2�����ʵ�����Ȼ���ټ����Ũ�ȡ�
(1)����ͼ��֪SCR������NH3��NO��NO2��Ӧ����ΪN2��ˮ����������ΪNO��NO2��
��NH3�е��Ļ��ϼ���-3�����ߵ�0�ۣ�һ��NH3ʧȥ3�����ӣ�NO2�е��Ļ��ϼ���+4�۽��͵�0�ۣ�һ��NO2�õ�4�����ӣ�NO�е��Ļ��ϼ���+2�۽��͵�0�ۣ�һ��NO�õ�2�����ӣ���NO2��NO�����ʵ���֮��Ϊ1��1ʱ��ת�Ƶ��ӵ���С������Ϊ6����������غ��֪����ʽΪ��2NH3+NO+NO2![]() 2N2+3H2O��
2N2+3H2O��
(2)�ټ���һ���� Na2S2O3��Һ��Na2S2O3��I2����������ԭ��ӦΪ��2S2O32-+I2=S4O62-+2I-��ʹI2��ԭΪI-������Ӧ��ȫ������Һ��ɫ����ʧ��������ڲ���ɫ��
����ͼʾ������pH��1��3ʱ��ClO2-��I-�������ɵ�I2����Һ�ֱ�Ϊ��ɫ��ClO2-���ȵĻ��ϼ���+3�۽��͵�-1�ۣ�һ��ClO2-�õ�4�����ӣ���Ļ��ϼ���-1�����ߵ�0�ۣ�һ��I-ʧȥ1�����ӣ�ת�Ƶ��ӵ���С������Ϊ4����������غ㡢����غ��֪���ӷ���ʽΪ��ClO2-+4I-+4H+=Cl-+2I2+2H2O��
�۸��ݵ��ӵ�ʧ��Ŀ��ȣ��ɵù�ϵʽ��ClO2��I-��S2O32-��n(S2O32-)=1.0��10-3 mol/L��0.01 L=1.0��10-5 mol����n(ClO2)=n(S2O32-)=1.0��10-5 mol����m(ClO2)= 1.0��10-5 mol��67.5��103 mg/mol=0.675 mg������ˮ�������Ϊ1.0 L������ ClO2 ��Ũ��Ϊ![]() =0.675 mg/L��
=0.675 mg/L��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����������( )��һ�ָ�Ч�������������ڻ�������ɱ��������Ѹ��ɱ��������������ɱ��������������Ũ������������Ƶã�ʵ��װ�úͲ������£�
)��һ�ָ�Ч�������������ڻ�������ɱ��������Ѹ��ɱ��������������ɱ��������������Ũ������������Ƶã�ʵ��װ�úͲ������£�
����������ƿ�м���һ������������ŨH2SO4�Ļ��Һ�壬�ٻ�����������30%��˫��ˮ��
�ڲ��Ͻ��貢����B�л��Һ���¶�Ϊ20��30������Ӧ������
�۽��������ܺͳ����ã�����ƿ���ռ��õ���Ʒ��

��ش��������⣺
(1)����B��������__������C����ˮ�������__(��a��b)��
(2)Ϊ���õؿ��Ʒ�Ӧ�¶ȣ�Ӧ���÷�����__��
(3)���ɹ�������Ļ�ѧ����ʽΪ__��
(4)��ͬ��Ӧ�������ʵ�������ɹ������Ậ��(%)��ʱ��ı仯����(���±�)���ɱ������ݿ�֪����Ӧ����ѱ���(CH3COOH/H2O2)��__����Ӧ����ʱ��Լ__(ѡ��1��3��5��7)Сʱ��
��Ӧ�����CH3COOH/H2O2 | ��Ӧʱ��(Сʱ) | ||||
0.5 | 1 | 3 | 5 | 7 | |
2��1 | 7.38 | 8.46 | 9.42 | 11.26 | 13.48 |
1��1 | 10.56 | 12.92 | 13.54 | 20.72 | 20.70 |
1��2 | 6.14 | 7.10 | 7.96 | 10.38 | 12.36 |
(5)�����ʵ��Ƚ�Fe3+��Cu2+�Թ�������Ĵ�Ч�ʣ��ɹ�ѡ����Լ�����Ҫ�����У�a.����������Һ��b.1mol/L��FeCl3��Һ��c.0.5mol/L��Fe2(SO4)3��Һ��d.0.5mol/L��CuCl2��Һ��e.1mol/L��CuSO4��Һ��f.��ʱ����g.���������������Ͳ��i.�������ܵ��Թܡ�
��ѡ����Լ���������a��__��f
(6)������ȡ2.00mL������������ϡ�ͳ�100mL������ȡ��5.00mL���μ�����KMnO4��Һ��ǡ�÷ۺ�ɫ�Գ�ȥ����H2O2���ټ���10mL10%KI��Һ�ͼ��ε�����Һ��ҡ�ȣ���Ӧ��ȫ������0.1000mol/L��Na2S2O3��Һ�ζ����յ�(��Ӧ����ʽΪ2Na2S2O3+I2=Na2S4O6+2NaI)��������14.30mLNa2S2O3��Һ������Ʒ�й�����������ʵ���Ũ����__mol/L��(�������С�������λ)
��ʾ��CH3COOOH+2I-+2H+=I2+CH3COOH+H2O)