��Ŀ����
�����Ϊ1L�������м���1mol N2��6mol H2���п��淴Ӧ��N2+3H2  2NH3��2min����N2�����ʵ���Ϊ0.6mol����
2NH3��2min����N2�����ʵ���Ϊ0.6mol����
��1��2min�ڣ�N2�����ʵ���������0.4mol��H2�����ʵ���������
��2������N2��Ũ�ȱ仯����ʾ�÷�Ӧ�ķ�Ӧ���ʣ���v��N2��=
��3������H2��Ũ�ȱ仯����ʾ�÷�Ӧ�ķ�Ӧ���ʣ���v��H2��=
��4������NH3��Ũ�ȱ仯����ʾ�÷�Ӧ�ķ�Ӧ���ʣ���v��NH3��=
��5��ͨ���������㣬����ʲô���֣�
 2NH3��2min����N2�����ʵ���Ϊ0.6mol����
2NH3��2min����N2�����ʵ���Ϊ0.6mol������1��2min�ڣ�N2�����ʵ���������0.4mol��H2�����ʵ���������
1.2
1.2
mol��NH3�����ʵ���������0.8
0.8
mol����2������N2��Ũ�ȱ仯����ʾ�÷�Ӧ�ķ�Ӧ���ʣ���v��N2��=
0.2
0.2
mol/��L?min������3������H2��Ũ�ȱ仯����ʾ�÷�Ӧ�ķ�Ӧ���ʣ���v��H2��=
0.6
0.6
mol/��L?min������4������NH3��Ũ�ȱ仯����ʾ�÷�Ӧ�ķ�Ӧ���ʣ���v��NH3��=
0.4
0.4
mol/��L?min������5��ͨ���������㣬����ʲô���֣�
ͬһ��ѧ��Ӧ�У������ʵķ�Ӧ����֮�ȵ����������֮�ȣ�
ͬһ��ѧ��Ӧ�У������ʵķ�Ӧ����֮�ȵ����������֮�ȣ�
����������1������N2�����ʵ����ı仯����Ϸ�Ӧ����ʽ����H2��NH3�����ʵ����ı仯����
��2������3������4������ƽ����ѧ��Ӧ���ʹ�ʽ��������ʵ�ƽ����Ӧ���ʣ�
��5��ͨ��������2������3������4��������ֵ֮���뻯ѧ������֮�����Ƚϣ��Ӷ��ó����ۣ�
��2������3������4������ƽ����ѧ��Ӧ���ʹ�ʽ��������ʵ�ƽ����Ӧ���ʣ�
��5��ͨ��������2������3������4��������ֵ֮���뻯ѧ������֮�����Ƚϣ��Ӷ��ó����ۣ�
����⣺��1��N2 +3 H2 ?2NH3
1 3 2
��Ӧ 0.4mol 1.2 mol 0.8mol
�ʴ�Ϊ��1.2 mol��0.8mol
��2��v��N2��=
=
=0.2 mol/��L?min����
�ʴ�Ϊ��0.2mol/��L?min����
��3��v��H2��=
=
=0.6mol/��L?min����
�ʴ�Ϊ��0.6mol/��L?min����
��4��v��NH3��=
=
=0.4mol/Lmin
�ʴ�Ϊ��0.4mol/Lmin
��5��v��N2����v��H2����v��NH3��=0.2 mol/��L?min����0.6 mol/��L?min����0.4mol/Lmin=1��3��2
ͨ���������֣�ͬһ��ѧ��Ӧ�У������ʵķ�Ӧ����֮�ȵ����������֮�ȣ�
1 3 2
��Ӧ 0.4mol 1.2 mol 0.8mol
�ʴ�Ϊ��1.2 mol��0.8mol
��2��v��N2��=
| ��n |
| V��t |
| 0.4mol |
| 1L2min |
�ʴ�Ϊ��0.2mol/��L?min����
��3��v��H2��=
| ��n |
| V��t |
| 1.2mol |
| 1L2min |
�ʴ�Ϊ��0.6mol/��L?min����
��4��v��NH3��=
| ��n |
| V��t |
| 0.8mol |
| 1L2min |
�ʴ�Ϊ��0.4mol/Lmin
��5��v��N2����v��H2����v��NH3��=0.2 mol/��L?min����0.6 mol/��L?min����0.4mol/Lmin=1��3��2
ͨ���������֣�ͬһ��ѧ��Ӧ�У������ʵķ�Ӧ����֮�ȵ����������֮�ȣ�
���������⿼����ǻ�ѧ��Ӧ���ʵĶ�����ʾ���������ؿ���ѧ���ķ�������������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
��һ���Ϊ1L�������У�ͨ��һ������N2O4
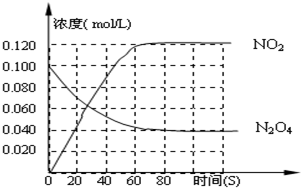
����100��ʱ�������·�Ӧ��N2O4 2NO2�CQ��Q>0������N2O4 ��NO2 Ũ�ȱ仯��ͼ��ʾ��
2NO2�CQ��Q>0������N2O4 ��NO2 Ũ�ȱ仯��ͼ��ʾ��

��1�� ������Ӧ��ƽ�ⳣ������ʽΪ______________�������¶�Kֵ_______�����������С�����䡱��
��2�� ��0-60s���ʱ���ڣ�������������ƽ����Ӧ����Ϊ________mol/L��s
��3��120��ʱ������ͬ�������з���������Ӧ�������ڸ����ʵ����ʵ����仯��ͼ��
 |
�ٸ��¶�ʱ����Ӧ����ƽ��״̬��ʱ����____________��C1����ֵ_____0��04������ڡ�����С�ڡ����ڡ���
�ڷ�Ӧ��60-80s��ƽ�����淴Ӧ�����ƶ������ܵ�ԭ���ǣ� ��
��A�� ʹ�ô��� ��B�� ����N2O4��Ũ��
��C����С��ϵѹǿ ��D�� ����NO2��Ũ��
 ��һ���Ϊ1L�������У�ͨ��һ������N2O4����100��ʱ�������·�Ӧ��N2O4
��һ���Ϊ1L�������У�ͨ��һ������N2O4����100��ʱ�������·�Ӧ��N2O4  2NO2-Q��Q��0������N2O4 ��NO2 Ũ�ȱ仯��ͼ��ʾ��
2NO2-Q��Q��0������N2O4 ��NO2 Ũ�ȱ仯��ͼ��ʾ��

 2NH3��2min����N2�����ʵ���Ϊ0.6mol����
2NH3��2min����N2�����ʵ���Ϊ0.6mol����