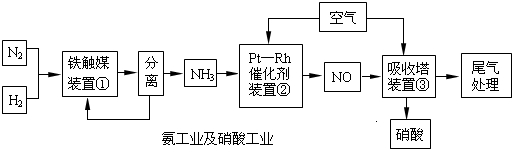
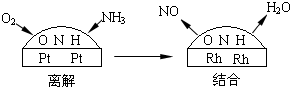
��Ŀ����
 ��ҵ�ϳɰ����Ʊ�����һ��������������������£�
��ҵ�ϳɰ����Ʊ�����һ��������������������£�

��1���ϳ����з�����Ӧ��N2��g��+3H2��g��?2NH3��g��+Q��Q��0�����±�Ϊ��ͬ�¶��¸÷�Ӧ��ƽ�ⳣ����
| T/�� | T1 | 300 | T2 |
| K | 1.00��107 | 2.45��105 | 1.88��103 |
��2��NH3��O2�ڲ�ϵ���������´�145��Ϳ�ʼ��Ӧ��
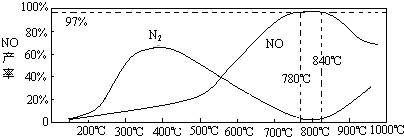
4NH3��g��+5O2��g��?4NO��g��+6H2O��g��+905kJ����ͬ�¶���NO����IJ�����ͼ��ʾ���¶ȸ���900��ʱ��NO��������½���ԭ����______��
��3���������з�ӦΪ��3NO2+H2O?2HNO3+NO�����������̿�������������Ҫ�����������ԭ����______��
��4�����᳧��β�����е��������������ֱ���ŷŽ���Ⱦ������Ŀǰ��ѧ��̽������ȼ�������еļ���Ƚ��������ﻹԭΪ������ˮ���䷴Ӧ����Ϊ��
CH4��g��+4NO2��g����4NO��g��+CO2��g��+2H2O��g��+574kJ��
CH4��g��+4NO��g����2N2��g��+CO2��g��+2H2O��g��+1160kJ��
��1mol����ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪ��______��
�⣺��1������N2��g��+3H2��g��?2NH3��g��+Q��Q��0����֪����Ӧ�Ƿ��ȷ�Ӧ���¶�Խ�ߣ�ƽ�ⳣ��ԽС������T2��300�棬�ʴ�Ϊ������
��2���¶���900��ʱ����Ӧ�ﵽƽ��״̬���¶ȸ���900��ʱ���������¶ȣ�ƽ�������ȷ��������ƶ����ʴ�Ϊ���¶ȸ���900��ʱ��ƽ�������ƶ���
��3������������Ҫ���������������NOת��ΪNO2�����ԭ�������ʣ��ʴ�Ϊ�����������NOת��ΪNO2�����������ԭ�������ʣ�
��4����֪����CH4��g��+4NO2��g����4NO��g��+CO2��g��+2H2O��g��+574kJ��
��CH4��g��+4NO��g����2N2��g��+CO2��g��+2H2O��g��+1160kJ��
����ֱ�ӽ�NO2��ԭΪN2�Ļ�ѧ����ʽ��CH4+2NO2=CO2+N2�������� ����+�ڣ��õ�������1mol����ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪCH4��g��+2NO2��g��=CO2��g��+N2��g����H=+867kJ/mol���ʴ�Ϊ��CH4��g��+2NO2��g��=CO2��g��+N2��g����H=+867kJ/mol��
����+�ڣ��õ�������1mol����ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪCH4��g��+2NO2��g��=CO2��g��+N2��g����H=+867kJ/mol���ʴ�Ϊ��CH4��g��+2NO2��g��=CO2��g��+N2��g����H=+867kJ/mol��
��������1�������¶ȶԻ�ѧƽ�ⳣ����Ӱ��ͷ�Ӧ������������йأ�
��2�����ڷ��ȷ�Ӧ���¶����ߣ���ѧƽ���������ƶ���
��3��һ���������Ժ�����������Ӧ���ɶ���������
��4�����ݸ�˹���������㷴Ӧ���ʱ䣬������д�Ȼ�ѧ����ʽ��
������������һ����ҵ�ϳɰ����ۺ���֪ʶ��Ŀ�����Ը�����ѧ֪ʶ���лش��ѶȽϴ�
��2���¶���900��ʱ����Ӧ�ﵽƽ��״̬���¶ȸ���900��ʱ���������¶ȣ�ƽ�������ȷ��������ƶ����ʴ�Ϊ���¶ȸ���900��ʱ��ƽ�������ƶ���
��3������������Ҫ���������������NOת��ΪNO2�����ԭ�������ʣ��ʴ�Ϊ�����������NOת��ΪNO2�����������ԭ�������ʣ�
��4����֪����CH4��g��+4NO2��g����4NO��g��+CO2��g��+2H2O��g��+574kJ��
��CH4��g��+4NO��g����2N2��g��+CO2��g��+2H2O��g��+1160kJ��
����ֱ�ӽ�NO2��ԭΪN2�Ļ�ѧ����ʽ��CH4+2NO2=CO2+N2��������
 ����+�ڣ��õ�������1mol����ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪCH4��g��+2NO2��g��=CO2��g��+N2��g����H=+867kJ/mol���ʴ�Ϊ��CH4��g��+2NO2��g��=CO2��g��+N2��g����H=+867kJ/mol��
����+�ڣ��õ�������1mol����ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪCH4��g��+2NO2��g��=CO2��g��+N2��g����H=+867kJ/mol���ʴ�Ϊ��CH4��g��+2NO2��g��=CO2��g��+N2��g����H=+867kJ/mol����������1�������¶ȶԻ�ѧƽ�ⳣ����Ӱ��ͷ�Ӧ������������йأ�
��2�����ڷ��ȷ�Ӧ���¶����ߣ���ѧƽ���������ƶ���
��3��һ���������Ժ�����������Ӧ���ɶ���������
��4�����ݸ�˹���������㷴Ӧ���ʱ䣬������д�Ȼ�ѧ����ʽ��
������������һ����ҵ�ϳɰ����ۺ���֪ʶ��Ŀ�����Ը�����ѧ֪ʶ���лش��ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
��ҵ�ϳɰ����Ʊ�����һ�������������������ͼ��

��1����ҵ����ʱ����ȡ������һ����ӦΪ��CO��g��+H2O��g��?CO2��g��+H2��g����t��ʱ����10L�ܱ������г���2mol CO��3molˮ��������Ӧ����ƽ�����ϵ��c��H2��=0.12mol?L-1������¶��´˷�Ӧ��ƽ�ⳣ��K= �������������
��2���ϳ����з�����ӦN2��g��+3H2��g��?2NH3��g����H��0���±�Ϊ��ͬ�¶��¸÷�Ӧ��ƽ�ⳣ�����ɴ˿���֪������T1 300�棨���������������=������
��3�������ڴ�����ȼ������һ�ֵ��ʺ�ˮ����ѧ�����ô�ԭ������Ƴɡ�����-������ȼ�ϵ�أ���ͨ�백���ĵ缫�� ����������������������������£��õ缫������Ӧ�ĵ缫��ӦʽΪ ��
��4���ð������������������ᣬ��β���е�NOx����Ⱦ������Ŀǰ��ѧ��̽������ȼ�������еļ���Ƚ����������ﻹԭΪ������ˮ����Ӧ����Ϊ��
CH4��g��+4NO2��g���T4NO��g��+CO2��g��+2H2O��g����H=-574kJ?mol-1
CH4��g��+4NO��g���T2N2��g��+CO2��g��+2H2O��g����H=-1160kJ?mol-1
�����ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪ ��
��5��ij�о�С����ʵ�����ԡ�Ag-ZSM-5��Ϊ��������ý�NOת��ΪN2��ת�������¶ȱ仯�������ͼ����ͼ����������ʹ��CO���¶ȳ���775�棬����NO��ת���ʽ��ͣ�����ܵ�ԭ��Ϊ ����
=1�������£�Ӧ���Ƶ�����¶��� ���ң�


��1����ҵ����ʱ����ȡ������һ����ӦΪ��CO��g��+H2O��g��?CO2��g��+H2��g����t��ʱ����10L�ܱ������г���2mol CO��3molˮ��������Ӧ����ƽ�����ϵ��c��H2��=0.12mol?L-1������¶��´˷�Ӧ��ƽ�ⳣ��K=
��2���ϳ����з�����ӦN2��g��+3H2��g��?2NH3��g����H��0���±�Ϊ��ͬ�¶��¸÷�Ӧ��ƽ�ⳣ�����ɴ˿���֪������T1
| T/�� | T1 | 300 | T2 |
| K | 1.00��107 | 2.45��105 | 1.88��103 |
��4���ð������������������ᣬ��β���е�NOx����Ⱦ������Ŀǰ��ѧ��̽������ȼ�������еļ���Ƚ����������ﻹԭΪ������ˮ����Ӧ����Ϊ��
CH4��g��+4NO2��g���T4NO��g��+CO2��g��+2H2O��g����H=-574kJ?mol-1
CH4��g��+4NO��g���T2N2��g��+CO2��g��+2H2O��g����H=-1160kJ?mol-1
�����ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪ
��5��ij�о�С����ʵ�����ԡ�Ag-ZSM-5��Ϊ��������ý�NOת��ΪN2��ת�������¶ȱ仯�������ͼ����ͼ����������ʹ��CO���¶ȳ���775�棬����NO��ת���ʽ��ͣ�����ܵ�ԭ��Ϊ
| n(NO) |
| n(CO) |



 ��3��NH3��O2�ڲ�ϵ���������´�145��Ϳ�ʼ��Ӧ��4NH3��g��+5O2��g��?4NO��g��+6H2O��g����H=-905kJ?mol-1����ͬ�¶���NO��������ͼ��ʾ���¶ȸ���900��ʱ��NO�����½���ԭ��
��3��NH3��O2�ڲ�ϵ���������´�145��Ϳ�ʼ��Ӧ��4NH3��g��+5O2��g��?4NO��g��+6H2O��g����H=-905kJ?mol-1����ͬ�¶���NO��������ͼ��ʾ���¶ȸ���900��ʱ��NO�����½���ԭ��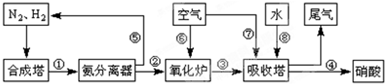
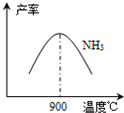 ��3��N2��H2���������������´�145��Ϳ�ʼ��Ӧ����ͬ�¶���NH3������ͼ��ʾ���¶ȸ���900��ʱ��NH3�����½���ԭ��
��3��N2��H2���������������´�145��Ϳ�ʼ��Ӧ����ͬ�¶���NH3������ͼ��ʾ���¶ȸ���900��ʱ��NH3�����½���ԭ��