��Ŀ����
�±�ΪԪ�����ڱ���һ���֣������г�10��Ԫ�������ڱ��е�λ�ã���Ҫ��ش����и��⡣

��1����10��Ԫ���У���ѧ��������õ�Ԫ����__________��д��ţ����õ���������ǿ��ԭ����
_______��дԪ�ط��ţ���ʧ����������ǿ�ĵ�����ˮ��Ӧ�Ļ�ѧ����ʽ��________________________��
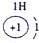
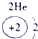
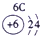
��2��Ԫ�آܵ����ӽṹʾ��ͼΪ____________��
��3���ߡ�������Ԫ������������Ӧˮ����Ļ�ѧʽ�ֱ�Ϊ��____________��____________
��4��д���۵ĵ����û����ĵ��ʵĻ�ѧ����ʽ________________________
��5���ٺ͢�����Ԫ������������Ӧ��ˮ�������Ӧ�����ӷ���ʽΪ��________________________
_______��дԪ�ط��ţ���ʧ����������ǿ�ĵ�����ˮ��Ӧ�Ļ�ѧ����ʽ��________________________��
��2��Ԫ�آܵ����ӽṹʾ��ͼΪ____________��
��3���ߡ�������Ԫ������������Ӧˮ����Ļ�ѧʽ�ֱ�Ϊ��____________��____________
��4��д���۵ĵ����û����ĵ��ʵĻ�ѧ����ʽ________________________
��5���ٺ͢�����Ԫ������������Ӧ��ˮ�������Ӧ�����ӷ���ʽΪ��________________________
��1���⣻F��2K+2H2O==2KOH+H2��
��2��
��3��H3PO4��HClO4
��4��2Mg+CO2 2MgO+C
2MgO+C
��5��Al(OH)3+OH-==AlO2-+2H2O
��2��

��3��H3PO4��HClO4
��4��2Mg+CO2
 2MgO+C
2MgO+C��5��Al(OH)3+OH-==AlO2-+2H2O

��ϰ��ϵ�д�
 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д�
�����Ŀ








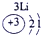
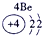
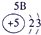
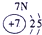
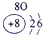
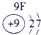
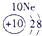
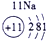





 ��ʾ����
��ʾ����