��Ŀ����
����ʯ����Կ�����MgO��Fe2O3��Al2O3��SiO2��ɡ�������ʯ��ȡ��ʽ̼��þ��ʵ�鲽������
| �������� | Fe(OH)3 | Al(OH)3 | Mg(OH)2 |
| ��ʼ����pH | 1.5 | 3.3 | 9.4 |
��2�����Т����ʱ��������ҺpH��7��8���й��������������pH���ϱ���
Ca(OH)2���ܹ�������Ca(OH)2�������ܻᵼ��_________�ܽ⡢_________������
��3���ӳ��������A����ȡ��ɫ�����������ϣ����������A�м���_________ (�������ʵĻ�ѧʽ)��Ȼ��__________________________________ (������дʵ���������)��
��4������ѭ��ʹ�ã��ܽ�Լ��Դ������ʵ���У�����ѭ��ʹ�õ�������_____________����д���ʻ�ѧʽ����
��5���������ռ�ʽ̼��þ�õ�MgO��ȡ��ʽ̼��þ4.66g���������������أ��õ�����2.00g�ͱ�״����CO20.896L��ͨ������ȷ����ʽ̼��þ�Ļ�ѧʽ ��
��6�����ȷֽⲻ��ȫ�����ü�ʽ̼��þ�н�����MgCO3�����Ʒ��þ�������������� �����ߡ��������͡����䡱����

����

 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�����ʯ����Կ�����MgO��Fe2O3��Al2O3��SiO2��ɡ�������ʯ��ȡ��ʽ̼��þ��ʵ�鲽�����£�
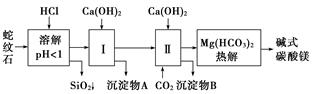
(1)����ʯ��������ܽ����Һ�����Mg2���⣬�����еĽ���������__________________��
(2)���Т����ʱ��������ҺpH��7��8(�й��������������pH���±�)��
|
�������� |
Fe(OH)3 |
Al(OH)3 |
Mg(OH)2 |
|
��ʼ����pH |
1.5 |
3.3 |
9.4 |
Ca(OH)2���ܹ�������Ca(OH)2�������ܻᵼ��________�ܽ⡢________������
(3)�ӳ��������A����ȡ��ɫ�����������ϣ����������A�м���_____________________ ___________________________________________________(�������ʵĻ�ѧʽ)��
Ȼ��______________________________________________________________________(������дʵ���������)��
(4)����ѭ��ʹ�ã��ܽ�Լ��Դ������ʵ���У�����ѭ��ʹ�õ�������________(��д���ʻ�ѧʽ)��
(5)�����һ��ʵ�飬ȷ����ƷaMgCO3��bMg(OH)2��cH2O��a��b��c��ֵ������������ʵ�鲽��(�����Լ���Ũ���ᡢ��ʯ��)��
����Ʒ����
�ڸ��·ֽ�
��________________________________________________________________________
��________________________________________________________________________
��MgO����
(6)18.2 g��Ʒ��ȫ�ֽ����6.6 g CO2��8.0 g MgO���ɴ˿�֪����Ʒ�Ļ�ѧʽ�У�
a��________��b��________��c��________��




