��Ŀ����

�й��������������pH���±���
| ���������� | �������↑ʼ����ʱ��pH | ����������ȫ����ʱ��pH |
| Fe3+ | 1.9 | 3.2 |
| Mg2+ | 9.4 | 11.6 |
��2���������е���pH��5��6ʱ�������õ����Լ�������ţ�
a�� NaOH��Һ b����ˮ c�� MgO d�� Mg ��OH��2
��3������ҺH��ȡ����Mg��������ͼ�ң�
������������ҺH�еõ�MgCl2?6H2O�ķ�����
���ڸ����HCl�����м���MgCl2?6H2O��ȡ��ˮ�Ȼ�þ��ԭ����
��ұ��þ�ķ����û�ѧ����ʽ��ʾΪ
| ||
| ���� |
| ||
| ���� |
��2���������м���������ܺ����ᷴӦ���Ҳ������µ����ʣ�����þ��������þ������ˮ���������ᷴӦ���������pH���ã�������ҺpH=5��6ʱ���ٽ�������ˮ�⣬��������������������ab�������µ����ʣ��ʴ�Ϊ��cd��
��3��������Һ�õ����壬Ӧ��������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����Ȳ������ʴ�Ϊ������Ũ������ȴ�ᾧ��
��þ������ˮ������������þ�������ӣ�����Mg2++2H2O?Mg��OH��2+2H+�����Ȼ��������м��ȸù���ʱ��������Ũ����������þ����ˮ������������þ��
�ʴ�Ϊ��Mg2++2H2O?Mg��OH��2+2H+��ͨ��HCl��ʹC��H+����������Mg2+��ˮ���Mg��OH��2��ȷ������MgCl2��
��þ�ǻ��ý�����Ӧ���õ���������εķ���ұ�������Թ�ҵ���õ�������Ȼ�þ�ķ���ұ��þ����Ӧ����ʽΪ��MgCl2
| ||
| ���� |
�ʴ�Ϊ��MgCl2
| ||
| ���� |

����ʯ����Կ�����MgO��Fe2O3��Al2O3��SiO2��ɡ�������ʯ��ȡ��ʽ̼��þ��ʵ�鲽�����£�
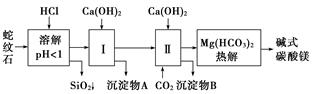
(1)����ʯ��������ܽ����Һ�����Mg2���⣬�����еĽ���������__________________��
(2)���Т����ʱ��������ҺpH��7��8(�й��������������pH���±�)��
|
�������� |
Fe(OH)3 |
Al(OH)3 |
Mg(OH)2 |
|
��ʼ����pH |
1.5 |
3.3 |
9.4 |
Ca(OH)2���ܹ�������Ca(OH)2�������ܻᵼ��________�ܽ⡢________������
(3)�ӳ��������A����ȡ��ɫ�����������ϣ����������A�м���_____________________ ___________________________________________________(�������ʵĻ�ѧʽ)��
Ȼ��______________________________________________________________________(������дʵ���������)��
(4)����ѭ��ʹ�ã��ܽ�Լ��Դ������ʵ���У�����ѭ��ʹ�õ�������________(��д���ʻ�ѧʽ)��
(5)�����һ��ʵ�飬ȷ����ƷaMgCO3��bMg(OH)2��cH2O��a��b��c��ֵ������������ʵ�鲽��(�����Լ���Ũ���ᡢ��ʯ��)��
����Ʒ����
�ڸ��·ֽ�
��________________________________________________________________________
��________________________________________________________________________
��MgO����
(6)18.2 g��Ʒ��ȫ�ֽ����6.6 g CO2��8.0 g MgO���ɴ˿�֪����Ʒ�Ļ�ѧʽ�У�
a��________��b��________��c��________��



