��Ŀ����
20��ʯ�ͻ�������Ҫԭ��CxHy���Ժϳɺܶ��л������������CxHy�ϳ�����E��J������ͼ��
��֪�������з�Ӧ��R��R���������

��J�ķ���ʽΪC4H4O4����һ�ֻ�״�����
��1����CxHy��ͬϵ���У�����̼ԭ��һ����ƽ����̼ԭ�������ķ��ӵ�������2��3-����-2-��ϩ��
��2��H�ķ���ʽ��C2H3O2Br��
��3������˵����ȷ����be��
a��CxHy�ͱ�����ʹ��ˮ��ɫ��ԭ����ͬ
b����Ӧ�ںͷ�Ӧ�ܵķ�Ӧ���;�Ϊ�ӳɷ�Ӧ
c��C����Na��NaOH��NaHCO3��Ӧ
d��E��һ��ˮ���Ժܺõĸ߷��ӻ�����
e��J�����Ի���Ի����о���ˮ��
��4��K��J��ͬ���칹�壬��1mol K��������NaHCO3��Һ��Ӧ�ɷų�2mol CO2���壬��д��һ�ַ�������K�Ľṹ��ʽCH2=C��COOH��2��HOOCCH=CHCOOH��
��5��д����Ӧ�ݵĻ�ѧ����ʽ2CH2OHCOOH

 +2H2O��
+2H2O����6��D�ж���ͬ���칹�壬��D������ͬ�����ŵĻ���5�֣���˳���칹�壩�����к˴Ź���������3�����շ壬���ܷ���������Ӧ�Ľṹ��ʽ��
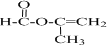 ��
��
���� J�ķ���ʽΪC4H4O4����һ�ֻ�״���������I��Ũ���������¼��ȵõ������Կ�����֪JΪ��������JΪ �������йط�Ӧ������������֪IΪHOCH2COOH��HΪBrCH2COOH��GΪBrCH2CHO��FΪBrCH2CH2OH��CxHy��HBrO�ӳɵ�F������CxHyΪCH2=CH2��������AΪCH3CHO��CH3CHO��������BΪCH3COOH��A��B������Ϣ�еļӳɷ�Ӧ��CΪCH3COOCH��OH��CH3��C��Ũ������������ˮ��DΪCH3COOCH=CH2��D�����Ӿ۷�Ӧ�õ�E��
�������йط�Ӧ������������֪IΪHOCH2COOH��HΪBrCH2COOH��GΪBrCH2CHO��FΪBrCH2CH2OH��CxHy��HBrO�ӳɵ�F������CxHyΪCH2=CH2��������AΪCH3CHO��CH3CHO��������BΪCH3COOH��A��B������Ϣ�еļӳɷ�Ӧ��CΪCH3COOCH��OH��CH3��C��Ũ������������ˮ��DΪCH3COOCH=CH2��D�����Ӿ۷�Ӧ�õ�E��
��� �⣺J�ķ���ʽΪC4H4O4����һ�ֻ�״���������I��Ũ���������¼��ȵõ������Կ�����֪JΪ��������JΪ �������йط�Ӧ������������֪IΪHOCH2COOH��HΪBrCH2COOH��GΪBrCH2CHO��FΪBrCH2CH2OH��CxHy��HBrO�ӳɵ�F������CxHyΪCH2=CH2��������AΪCH3CHO��CH3CHO��������BΪCH3COOH��A��B������Ϣ�еļӳɷ�Ӧ��CΪCH3COOCH��OH��CH3��C��Ũ������������ˮ��DΪCH3COOCH=CH2��D�����Ӿ۷�Ӧ�õ�E��
�������йط�Ӧ������������֪IΪHOCH2COOH��HΪBrCH2COOH��GΪBrCH2CHO��FΪBrCH2CH2OH��CxHy��HBrO�ӳɵ�F������CxHyΪCH2=CH2��������AΪCH3CHO��CH3CHO��������BΪCH3COOH��A��B������Ϣ�еļӳɷ�Ӧ��CΪCH3COOCH��OH��CH3��C��Ũ������������ˮ��DΪCH3COOCH=CH2��D�����Ӿ۷�Ӧ�õ�E��
��1����CxHy��ͬϵ���У�����̼ԭ��һ����ƽ����̼ԭ�������ķ���Ӧ���Ǻ���6��̼ԭ�ӵ�ϩ�����ṹ��ʽΪ��CH3��2C=C��CH3��2��������2��3-����-2-��ϩ��
�ʴ�Ϊ��2��3-����-2-��ϩ��
��2��H�Ľṹ��ʽΪBrCH2COOH������H�ķ���ʽ��C2H3O2Br��
�ʴ�Ϊ��C2H3O2Br��
��3��a����ϩ�ͱ�����ʹ��ˮ��ɫ��ԭ������ͬ��ǰ���Ǽӳɷ�Ӧ����������ȡ����a����
b���������Ϸ�����֪��Ӧ����ȩ���ļӳɷ�Ӧ����Ӧ��Ϊ̼̼˫���ļӳɷ�Ӧ����b��ȷ��
c���ǻ�������������NaHCO3��Ӧ����c����
d��E�����к�����������������һ��ˮ���Ժܺõĸ߷��ӻ������d����
e��J�к��������������Ի���Ի����о���ˮ�⣬��e��ȷ��
�ʴ�Ϊ��be��
��4��K��J�� ����ͬ���칹�壬��1mol K��������NaHCO3��Һ��Ӧ�ɷų�2mol CO2���壬��˵������2���Ȼ������������K�Ľṹ��ʽ�����ǣ�CH2=C��COOH��2��HOOCCH=CHCOOH��
����ͬ���칹�壬��1mol K��������NaHCO3��Һ��Ӧ�ɷų�2mol CO2���壬��˵������2���Ȼ������������K�Ľṹ��ʽ�����ǣ�CH2=C��COOH��2��HOOCCH=CHCOOH��
�ʴ�Ϊ��CH2=C��COOH��2��HOOCCH=CHCOOH��
��5���������Ϸ�����֪����Ӧ�ݵĻ�ѧ����ʽΪ��2CH2OHCOOH 
 +2H2O��
+2H2O��
�ʴ�Ϊ��2CH2OHCOOH 
 +2H2O��
+2H2O��
��6��D��CH3COOCH=CH2���ж���ͬ���칹�壬��D������ͬ�����ţ�������������̼̼˫����������������л���ṹ��ʽ�����ǣ�HCOOCH2CH=CH2��HCOOCH=CHCH3������˳���칹�壩��HCOOC��CH3��=CH2��CH2=CHCOOCH3��������5�֣����к˴Ź���������3�����շ壬���ܷ���������Ӧ�Ľṹ��ʽ��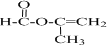 ��
��
�ʴ�Ϊ��5��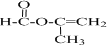 ��
��
���� ���⿼���л����ƶϺͺϳɡ��л����������л���ṹ�����ʡ�ͬ���칹���жϼ�����ʽ��д�ȣ����ؿ���ѧ�������ƶ����������������Ϣ����Ӧ�������������̼���仯�����ƶϼ��ɣ��������չ����ŵ�������ת������Ŀ�Ѷ��еȣ�

 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�| A | B | C | D | |
ͼʾ |  |  |  |  |
| ���� | HBΪ���� | HFΪ������� | �ܽ�ȣ�AgI��AgCl | ����Ӧ��H��0 |
| A�� | A | B�� | B | C�� | C | D�� | D |

����������ȷ���ǣ�������
| A�� | ����FeCl3��Һ������I��II | |
| B�� | ����I��NaOH����Һ�м��ȿɷ�����ȥ��Ӧ | |
| C�� | ����II������ԭ�ӿ���λ��ͬһƽ���� | |
| D�� | ����III������H2�ӳ����ò����������2������̼ԭ�� |
| A�� | �Ʊ�AlCl3��FeCl3��CuCl2�����ܲ��ý���Һֱ�����ɵķ��� | |
| B�� | ���ߵĵ��ʷ����ڿ����о�ֻ���������� | |
| C�� | ��ҵ�ϣ����ߵĵ���Ŀǰ��ʹ���Ȼ�ԭ���Ƶ� | |
| D�� | ���AlCl3��FeCl3��CuCl2�Ļ����Һʱ��������������Cu��Fe��Al |

��֪�����ֽ��������������������������pH��Χ���±���ʾ����ʼ������pH����������Ũ��Ϊ1.0mol/L���㣩
| ���� | ��ʼ������pH | ��ȫ������pH |
| Fe3+ | 1.1 | 3.2 |
| Mn2+ | 8.3 | 9.8 |
| Cu2+ | 4.4 | 6.4 |
��1����ȡ��õ��Ľ���Һ�к���CuSO4��MnSO4��д����ȡʱ����CuSO4��MnSO4��Ӧ�Ļ�ѧ����ʽ2MnO2+Cu2S+4H2SO4=S��+2CuSO4+2MnSO4+4H2O��
��2������pH��Ŀ����������ת��������������ȫ������pH�ĵ��ڷ�ΧΪ3.2��pH��4.4��
��3������MnCO3���������ӷ���ʽΪMn2++NH3+HCO3-=MnCO3��+NH4+��
��4������AΪ����Ũ������ȴ�ᾧ��
��5���ɻ�ͭ����ȡͭ�ķ�Ӧ���̿��Ա�ʾΪ��
2Cu2S��s��+3O2��g���T2Cu2O��s��+2SO2��g����H=-768.2kJ/mol
2Cu2O��s��+Cu2S��s���T6Cu��s��+SO2��g����H=+116.0kJ/mol
����Cu2S��O2���ȷ�Ӧ����Cu���Ȼ�ѧ����ʽΪCu2S��s��+02��g���T2Cu��s��+SO2��g����H=-217.4kJ��mol-l��
��6�����ú�85%Cu2S��Mr=160���Ļ�ͭ�����Ʊ���ˮCu��NO3��2�������ȡ��Ϊ95%������pHʱ��ʧCu3%��������������5%δת��ΪCuO����������������ģ���1.6kg�����Ļ�ͭ��������Ʊ�14.9mol��ˮCu��NO3��2������������ȷ��С�����1λ��
| A�� | H2��O2��Ӧ������H2O | B�� | Zn��Ͷ��ϡ������ | ||
| C�� | KMnO4������O2 | D�� | C��O2��Ӧ����CO2 |
| A�� | NaCl��Һ | B�� | �������� | C�� | ���� | D�� | ���� |
