��Ŀ����
5��ij����С���ͬѧ������̽��п����������ķ�Ӧ��i�������о�п��Ũ����ķ�Ӧ����ͬѧ��Ϊ�����������SO2�⣬�����ܲ�����������ͬѧΪ�˼��������ж��Ƿ���ȷ���������ͼ��ʾ��ʵ��װ�ã����У�װ��B��ʢ��Ũ���ᣬװ��C�з��ú�ɫCuO��ĩ��װ��D�з��õ�����ˮ����ͭ��ĩ��п��Ũ���Ṳ��ʱ��������ΪX���Ҹ�װ����ȥ�����Իش�
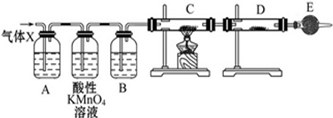
��1��������Ӧ�����ɶ�������Ļ�ѧ����ʽΪZn+2H2SO4 ��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$ZnSO4+SO2��+2H2O
��2��A�м�����Լ�������Ʒ����Һ
��3��E�м�����Լ��Ǽ�ʯ�ң������Ƿ�ֹ�����е�ˮ��������D�У����ż���
��4��װ��A��B֮������Ը��������Һ�������dz�ȥ����X�е�SO2
��5������֤������X�к���������ʵ�������Ǻ�ɫ��ĩ��CuO����ɺ�ɫ���������ʣ�D�а�ɫ��ĩ�����ɫ����
��ii������ͬѧ�о�п������ķ�Ӧ
��1������ͬѧ���ֵ������Ũ�ȼ���ʱ����Ӧ�������������п���������ĵ����ʵ���֮��Ϊ2��5����ԭ����ֻ��һ�֣�������Ļ�ԭ����ΪNH4NO3��N2O����Ӧ�Ļ�ѧ����ʽΪ4Zn+10HNO3=4Zn��NO3��2+NH4NO3+3H2O��4Zn+10HNO3=4Zn��NO3��2+N2O��+5H2O
��2������ͬѧ�ԱȲ�ͬŨ�ȵ�������п��Ӧ����IJ�ͬ�����Է�������Ũ��Խ�ߣ������е�Ԫ�ؼ�̬Խ�ߣ�
���� ��i��Ũ�������ǿ�����ԣ�����п���л�ԭ�ԣ�Zn��ŨH2SO4��Ӧ��������п�����������ˮ����Ӧ�ķ���ʽΪ��Zn+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$ZnSO4+SO2��+2H2O���淴Ӧ���У�Ũ�����Ϊϡ���ᣬZn��ϡ���ᷴӦ��������п��������װ��A������Ϊ��������X���ж�������ͨ��ʹ��Ʒ����Һ�����Ը��������Һ��ȥ��������Bװ������ˮ������C��������CuO�����û���Ӧ��D������ͭ��ˮ������C������ɫ�仯��D������ͭ��ɫ�仯���ж��Ƿ����������ɣ�E�п���ʢ�ż�ʯ�ң���ֹ������ˮ��������D�У�
��ii����1����Ӧ�����������������������ã��������������õ�������������п�����������ȫ�������������ã���ZnΪ2mol������Ϊ5mol������Znԭ���غ��������п�����ʵ����������������������õ����ᣬ���ݵ���ת���غ���㻹ԭ������NԪ�ػ��ϼۣ������жϻ�ԭ�����д��ѧ����ʽ��
��2�����ݣ�1������Ϣ��֪������Ũ��Խ�ߣ������е�Ԫ�ؼ�̬Խ�ߣ�
��� �⣺��i����1��Ũ�������ǿ�����ԣ�����п���л�ԭ�ԣ�Zn��ŨH2SO4��Ӧ��������п�����������ˮ����Ӧ�ķ���ʽΪ��Zn+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$ZnSO4+SO2��+2H2O��
�ʴ�Ϊ��Zn+2H2SO4 ��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$ZnSO4+SO2��+2H2O��
��2��Aװ�õ������Ǽ����������Ĵ��ڣ�����������Ư��Ʒ�죬����ͨ��Ʒ����Һ��ɫ����SO2�Ĵ��ڣ�
�ʴ�Ϊ��Ʒ����Һ��
��3�����Ը���D������ͭ��ˮ���ɰ�ɫ��ĩ�����ɫ���壬�ж����������ɣ�E�м�ʯ�����տ����е�ˮ��������ֹ�����е�ˮ��������D�У����ż��飬
�ʴ�Ϊ����ֹ�����е�ˮ��������D�У����ż��飻
��4��A��Bװ��֮������Ը��������Һ���ڳ�ȥ����X�еĶ����������壬
�ʴ�Ϊ����ȥ����X�е�SO2��
��5��C������������ͭ�ڼ��������·�Ӧ����ͭ��ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ��H2+CuO$\frac{\underline{\;\;��\;\;}}{\;}$Cu+H2O����ɫCuO��ɺ�ɫ��Cu��D��ˮ����ˮ����ͭ��Ӧ������ɫ����ˮ������ͭ����ɫ��ĩ��Ϊ��ɫ��֤������X�к���H2��ʵ�������ǣ�C�к�ɫCuO��ɺ�ɫ��Cu��D�а�ɫ��ĩ�����ɫ���壬
�ʴ�Ϊ����ɫ��ĩ��CuO����ɺ�ɫ���������ʣ�D�а�ɫ��ĩ�����ɫ���壻
��ii����1����Ӧ�����������������������ã��������������õ�������������п�����������ȫ�������������ã���ZnΪ2mol������Ϊ5mol������Znԭ���غ㣬��������пΪ2mol����NԪ���ڻ�ԭ�����еĻ��ϼ�Ϊa�����ݵ���ת���غ��У�2mol��2=��5mol-2mol��2������5-a�������a=1���ʻ�ԭ����ΪNH4NO3��N2O���÷�Ӧ����ʽΪ��4Zn+10HNO3=4Zn��NO3��2+NH4NO3+3H2O��4Zn+10HNO3=4Zn��NO3��2+N2O��+5H2O��
�ʴ�Ϊ��NH4NO3��N2O��4Zn+10HNO3=4Zn��NO3��2+NH4NO3+3H2O��4Zn+10HNO3=4Zn��NO3��2+N2O��+5H2O��
��2�����ݣ�1���е������Ũ�ȼ���ʱ����Ӧ�������������֪����Ũ��Խ�ߣ������е�Ԫ�ؼ�̬Խ�ߣ�
�ʴ�Ϊ���ߣ�
���� ���⿼��п��Ũ����ķ�Ӧ�����̽�����顢������ԭ��Ӧ�йؼ��㣬��Ŀ�Ѷ��еȣ���i���ؼ��������������ԭ������ii����ע�����õ���ת���غ�����跨�жϻ�ԭ����Ѷ��еȣ�

| ���� ��� | �� | �� | �� |
| A | SO2 | Ba��OH��2 | NaHCO3 |
| B | Na2O2 | H2O | CO2 |
| C | Na2SiO3 | NaOH | HCl |
| D | Al | H2SO4 | NaOH |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | 600mL 18.4mol/L H2SO4 | B�� | 100mL 1mol/L HCl | ||
| C�� | 200mL 80% HNO3 | D�� | 600mL 0.1mol/L HNO3 |
| A�� | H+��Cu2+��Fe3+��SO42- | B�� | Na+��Ba2+��Al3+��Cl- | ||
| C�� | K+��Ag+��NH4+��NO3- | D�� | Na+��K+��Br-��OH- |
| A�� | ���� | B�� | ���� | C�� | ��С | D�� | ���ж� |
| A�� | K2SO3�Ѳ��ֱ������е��������� | |
| B�� | ����Ba��NO3��2��Һ�����ɵij�����һ������BaSO4 | |
| C�� | �������IJ����Գ����к���BaSO3 | |
| D�� | ��ʵ�鲻��ȷ��K2SO3�Ƿֱ����� |
| A�� | N2���������������ǻ�ԭ�� | |
| B�� | NaNO2�������� | |
| C�� | NH4Cl�еĵ�Ԫ�ر���ԭ | |
| D�� | ÿ����1 mol N2ʱ��ת�Ƶ��ӵ����ʵ���Ϊ6 mol |
 ������أ�K2FeO4����һ����ɫ������������������չ�ֳ�������Ӧ��ǰ����
������أ�K2FeO4����һ����ɫ������������������չ�ֳ�������Ӧ��ǰ����