��Ŀ����
�Ի�ͭ��Ϊԭ�ϣ���ȡ����ͭ�Ĺ���������ʾ��

����ͭ����Ҫ�ɷ�ΪCuFeS2����������CaO��MgO��Al2O3�����顣
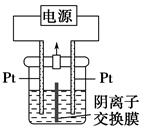
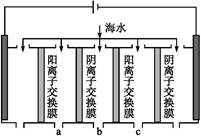
������ͼ��ʾװ�ý��е绯ѧ����ʵ�顣����ѡ��ͭ��ۼ�����۵������������ٽ��裬ʹ����ܽ⡣��������ͨ������������������������
��һ��ʱ���ȡ��������Һ�������м����л���ȡ��(RH)������Ӧ��2RH(�л���)+Cu2+(ˮ��) R2Cu(�л���)+2H��(ˮ��)������л��࣬�����м���һ��Ũ�ȵ����ᣬʹCu2+���������� �����������ͭ��Һ�Ƶý���ͭ��
R2Cu(�л���)+2H��(ˮ��)������л��࣬�����м���һ��Ũ�ȵ����ᣬʹCu2+���������� �����������ͭ��Һ�Ƶý���ͭ��
��1����ͭ��ۼ��������������ἰ��������Ҫ�������·�Ӧ��CuFeS2 + 4H+��Cu2+ + Fe2+ + 2H2S�� 2Fe3+ + H2S��2Fe2+ + S��+ 2H+ ������������������Ҫ������ ����2�����������缫�Ͽ�ʼʱ�д������ݲ��������к�ɫ����������һ��ʱ����ɫ�����ܽ⡣д��������ɫ����ķ�Ӧ����ʽ ��
��3������ʵ���ҽ��в�������л����ˮ�����Ҫʵ�������� ��
��4����������л����м���һ��Ũ�ȵ����ᣬCu2+����������ԭ���� ����5��������������200mL0.5 mol/L��CuSO4��Һ������ͭ3.2 g����ʱ��Һ������Ũ���ɴ�С��˳���� ��

����ͭ����Ҫ�ɷ�ΪCuFeS2����������CaO��MgO��Al2O3�����顣
������ͼ��ʾװ�ý��е绯ѧ����ʵ�顣����ѡ��ͭ��ۼ�����۵������������ٽ��裬ʹ����ܽ⡣��������ͨ������������������������
��һ��ʱ���ȡ��������Һ�������м����л���ȡ��(RH)������Ӧ��2RH(�л���)+Cu2+(ˮ��)
 R2Cu(�л���)+2H��(ˮ��)������л��࣬�����м���һ��Ũ�ȵ����ᣬʹCu2+���������� �����������ͭ��Һ�Ƶý���ͭ��
R2Cu(�л���)+2H��(ˮ��)������л��࣬�����м���һ��Ũ�ȵ����ᣬʹCu2+���������� �����������ͭ��Һ�Ƶý���ͭ����1����ͭ��ۼ��������������ἰ��������Ҫ�������·�Ӧ��CuFeS2 + 4H+��Cu2+ + Fe2+ + 2H2S�� 2Fe3+ + H2S��2Fe2+ + S��+ 2H+ ������������������Ҫ������ ����2�����������缫�Ͽ�ʼʱ�д������ݲ��������к�ɫ����������һ��ʱ����ɫ�����ܽ⡣д��������ɫ����ķ�Ӧ����ʽ ��
��3������ʵ���ҽ��в�������л����ˮ�����Ҫʵ�������� ��
��4����������л����м���һ��Ũ�ȵ����ᣬCu2+����������ԭ���� ����5��������������200mL0.5 mol/L��CuSO4��Һ������ͭ3.2 g����ʱ��Һ������Ũ���ɴ�С��˳���� ��
��1�������������壬��ֹ������Ⱦ�������������ɵ÷֣� ��1�֣�
��2��Cu2++2e�C��Cu ��2�֣� ��3����Һ©�� ��1�֣�
��4������H+Ũ�ȣ�ʹƽ��2RH(�л���) + Cu2+(ˮ��) R2Cu(�л���)+ 2H��(ˮ��)�����ƶ���Cu2+����ˮ��������� ��2�֣� ��5��c(H+)��c(SO42�C)��c(Cu2+)��c(OH�C) ��2�֣�
R2Cu(�л���)+ 2H��(ˮ��)�����ƶ���Cu2+����ˮ��������� ��2�֣� ��5��c(H+)��c(SO42�C)��c(Cu2+)��c(OH�C) ��2�֣�
��2��Cu2++2e�C��Cu ��2�֣� ��3����Һ©�� ��1�֣�
��4������H+Ũ�ȣ�ʹƽ��2RH(�л���) + Cu2+(ˮ��)
 R2Cu(�л���)+ 2H��(ˮ��)�����ƶ���Cu2+����ˮ��������� ��2�֣� ��5��c(H+)��c(SO42�C)��c(Cu2+)��c(OH�C) ��2�֣�
R2Cu(�л���)+ 2H��(ˮ��)�����ƶ���Cu2+����ˮ��������� ��2�֣� ��5��c(H+)��c(SO42�C)��c(Cu2+)��c(OH�C) ��2�֣������������1����ӦCuFeS2 + 4H+��Cu2+ + Fe2+ + 2H2S������H2S���������H2S�ж����ڴ�����Ⱦ����ݷ�Ӧ2Fe3+ + H2S��2Fe2+ + S��+ 2H+ ��֪������������������Ҫ�����������������壬��ֹ������Ⱦ��
��2�������������õ����ӣ�������ԭ��Ӧ������������ɫ���壬˵���ú�ɫ������ͭ����˵缫��ӦʽΪCu2++2e�C��Cu��
��3���л��ﲻ����ˮ����Һ���ɣ����Է����л����ˮ�����Ҫʵ�������Ƿ�Һ©����
��4���������ᣬ������Һ��H+Ũ�ȣ�ʹƽ��2RH(�л���) + Cu2+(ˮ��)
 R2Cu(�л���)+ 2H��(ˮ��)�����ƶ���Cu2+����ˮ�����������
R2Cu(�л���)+ 2H��(ˮ��)�����ƶ���Cu2+����ˮ�������������5��200mL0.5 mol/L��CuSO4��Һ������ͭ�����ʵ�����0.2L��0.5mol/L��0.1mol������ͭ3.2 g�����ʵ�����3.2g��64g/mol��0.05mol����˵����Ӧ������ͭ����0.05molû�б���⡣���ݷ���ʽ2CuSO4��2H2O
 2Cu��2H2SO4��O2����֪����Һ�л�����0.05mol���ᡣ����ͭ����ˮ�⣬����Һ������Ũ�ȴ�С˳���ǣ�c(H+)��c(SO42�C)��c(Cu2+)��c(OH�C)��
2Cu��2H2SO4��O2����֪����Һ�л�����0.05mol���ᡣ����ͭ����ˮ�⣬����Һ������Ũ�ȴ�С˳���ǣ�c(H+)��c(SO42�C)��c(Cu2+)��c(OH�C)��
��ϰ��ϵ�д�
�����Ŀ
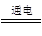 KIO3��3H2��
KIO3��3H2�� 

 H2 ��
H2 ��
