��Ŀ����
������Դ�Ŀ��������þ��й�����ǰ������ˮ��pHһ����7.5~8.6֮�䡣ij�غ�ˮ����Ҫ���ӵĺ������±�:
(1)��ˮ�������Ե�ԭ����(�����ӷ���ʽ��ʾ):����������������������,�ú�ˮ��Ca2+�����ʵ���Ũ��Ϊ���������� mol��L-1��
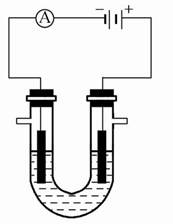
(2)���������ǽ��귢չ������һ�ֽϺõĺ�ˮ��������,��ԭ������ͼ��ʾ��������(��)���ӽ���Ĥֻ������(��)����ͨ����

������ԭ��ʾ��ͼ
�������ĵ缫��ӦΪ����������������������
�ڵ��һ��ʱ��,�����������ˮ��,��ɷ�ΪCaCO3��Mg(OH)2,д������CaCO3�����ӷ���ʽ:����������������������
�۵�ˮ�ij���Ϊa��b��c�е������������ڡ�
| �ɷ� | Na+ | K+ | Ca2+ | Mg2+ | Cl- | S | HC |
| ����/(mg��L-1) | 9 360 | 83 | 200 | 1 100 | 16 000 | 1 200 | 118 |
(1)��ˮ�������Ե�ԭ����(�����ӷ���ʽ��ʾ):����������������������,�ú�ˮ��Ca2+�����ʵ���Ũ��Ϊ���������� mol��L-1��
(2)���������ǽ��귢չ������һ�ֽϺõĺ�ˮ��������,��ԭ������ͼ��ʾ��������(��)���ӽ���Ĥֻ������(��)����ͨ����

������ԭ��ʾ��ͼ
�������ĵ缫��ӦΪ����������������������
�ڵ��һ��ʱ��,�����������ˮ��,��ɷ�ΪCaCO3��Mg(OH)2,д������CaCO3�����ӷ���ʽ:����������������������
�۵�ˮ�ij���Ϊa��b��c�е������������ڡ�
(1)HC +H2O
+H2O H2CO3+OH-��5��10-3
H2CO3+OH-��5��10-3
(2)��2H2O+2e- H2��+2OH-(��2H++2e-
H2��+2OH-(��2H++2e- H2��)
H2��)
��Ca2++OH-+HC
 CaCO3��+H2O����b
CaCO3��+H2O����b
 +H2O
+H2O H2CO3+OH-��5��10-3
H2CO3+OH-��5��10-3(2)��2H2O+2e-
 H2��+2OH-(��2H++2e-
H2��+2OH-(��2H++2e- H2��)
H2��)��Ca2++OH-+HC

 CaCO3��+H2O����b
CaCO3��+H2O����b(1)c(Ca2+)= ;(2)���������ұ������ƶ�������������������ƶ�,��֪b��Ϊ��ˮ
;(2)���������ұ������ƶ�������������������ƶ�,��֪b��Ϊ��ˮ
 ;(2)���������ұ������ƶ�������������������ƶ�,��֪b��Ϊ��ˮ
;(2)���������ұ������ƶ�������������������ƶ�,��֪b��Ϊ��ˮ
��ϰ��ϵ�д�
 ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д�
�����Ŀ


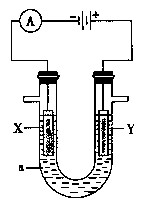

 R2Cu(�л���)+2H��(ˮ��)������л��࣬�����м���һ��Ũ�ȵ����ᣬʹCu2+���������� �����������ͭ��Һ�Ƶý���ͭ��
R2Cu(�л���)+2H��(ˮ��)������л��࣬�����м���һ��Ũ�ȵ����ᣬʹCu2+���������� �����������ͭ��Һ�Ƶý���ͭ��
