��Ŀ����
14��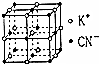 ��������������K3[Fe��CN��6]�׳Ƴ�Ѫ�Σ���ҽҩ��ҵ��ӡȾ��ҵ��������ҵ������Ҫ���ã���ش��������⣺
��������������K3[Fe��CN��6]�׳Ƴ�Ѫ�Σ���ҽҩ��ҵ��ӡȾ��ҵ��������ҵ������Ҫ���ã���ش��������⣺��1����Ѫ���������Ӿ��壬���������Ӽ۵����Ų�ʽΪ3d5��
��2��K3[Fe��CN��6]���������Ԫ�صĵ�һ�������ɴ�С��������N��C��Fe��K�����ڵ���������acd����ѡ����ţ���
a�����Ӽ� b�������� c����λ�� d�����Թ��ۼ� e���Ǽ��Թ��ۼ� f�����Ӽ�������
��3����±������SCN-������Fe3+�ļ��飬���Ӧ���������֣��ֱ�Ϊ�����ᣨH-S-C�TN�����������ᣨH-N�TC�TS�������������зе�ϸߵ����������ᣨ��H-N=C=S����
��4���������������س����º��ȶ��������տ���ȫ�ֽ⣬�����綾���軯�غ��裮
��ѧ����ʽ��2K3[Fe��CN��6]$\frac{\underline{\;����\;}}{\;}$6KCN+2FeC2+2N2+��CN��2
�ٲ����裨CH��2�к��еĦҼ��ͦм���Ŀ��Ϊ3��4��
�ڣ�CH��2��Cԭ�ӵ��ӻ��������Ϊsp����д��һ���루CH��2������ͬ�ռ乹�ͺ�ԭ�����ķ��ӣ�C2H2��
����KCN�ľ����ṹ��ͼ��ʾ����KCN�����о���K+�����K+����ĿΪ12�����þ�����ܶ�Ϊ�� g•cm-3��ͼʾ��K+��K+�ĺ˼��a=$\frac{\sqrt{2}}{2}��\root{3}{\frac{260}{��•{N}_{A}}}$cm��
���� ��1������K3[Fe��CN��6]����ɿ�֪�û�����Ϊ���ӻ������û�����Ϊ���Ӿ��壬����������Fe3+��Fe3+�ļ۵����Ų�ʽΪ3d5��
��2��ͬ�����������Ԫ�صĵ�һ�����ܳ��������ƣ�����Ԫ�ص�һ�����ܽ�С���ǽ���Ԫ�ص�һ�����ܽϴ�K3[Fe��CN��6]����λ�����Ҳ�����ӻ������û�������һ��������λ�������Ӽ���C��N֮����ڼ��Թ��ۼ���
��3������������H-N������ǿ�����Ӽ����������е�ϸߣ�
��4���ٲ��CN��2�еĽṹʽΪN��C-C��N���Ҽ���3�����м���4����
�ڲ��CN��2�еĽṹʽΪN��C-C��N������ԭ��C�¶Ե��ӣ���۲���Ӷ���Ϊ2���ӻ�����Ϊsp�ӻ����ռ乹��Ϊֱ���Σ�
�۴�ͼ�п�֪���������ϵ�K+Ϊ����ͬ�㶥���4��K+���������ϵ�K+������м�㻹��4�������Ҳ��4������12�����þ�̯�����㾧����K+��8��$\frac{1}{8}$+6��$\frac{1}{2}$=4��CN-��12��$\frac{1}{4}$+1=4��K+��K+�ĺ˼��a�������ⳤΪ$\sqrt{2}$a�����������V=��$\sqrt{2}$a��3�����ݾ���������m=��•V���㣮
��� �⣺��1������K3[Fe��CN��6]����ɿ�֪�û�����Ϊ���ӻ������û�����Ϊ���Ӿ��壬����������Fe3+��Fe��ԭ�Ӻ��������Ϊ26��ԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d64s2��ʧȥ3�������������������ӣ���Fe3+�ļ۵����Ų�ʽΪ3d5��
�ʴ�Ϊ�����ӣ�3d5��
��2��ͬ�����������Ԫ�صĵ�һ�����ܳ��������ƣ����һ������Fe��K����A��np�ܼ�����3�����ӣ�Ϊ������ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ���N��C������Ԫ�ص�һ�����ܽ�С���ǽ���Ԫ�ص�һ�����ܽϴ���K3[Fe��CN��6]���������Ԫ�صĵ�һ�����ɴ�С��������N��C��Fe��K��K3[Fe��CN��6]����λ�����Ҳ�����ӻ������û�������һ��������λ�������Ӽ���C��N֮����ڼ��Թ��ۼ������Դ��ڵ���������a��c��d��
�ʴ�Ϊ��N��C��Fe��K��acd��
��3������������H-N������ǿ�����Ӽ�������������������Ӽ�ֻ���ڷ��Ӽ���������������������ķе���������ᣬ
�ʴ�Ϊ���������ᣨ��H-N=C=S����
��4���ٲ��CN��2�еĽṹʽΪN��C-C��N���Ҽ���3�����м���4������Ҽ��ͦм���Ŀ֮��Ϊ3��4��
�ʴ�Ϊ��3��4��
�ڲ��CN��2�еĽṹʽΪN��C-C��N������ԭ��C�¶Ե��ӣ���۲���Ӷ���Ϊ2���ӻ�����Ϊsp�ӻ����ռ乹��Ϊֱ���Σ�
�����ռ乹�͡�ԭ������ͬ�ķ�����C2H2��H-C��C-H����
�ʴ�Ϊ��sp��C2H2��
�۴�ͼ�п�֪���������ϵ�K+Ϊ����ͬ�㶥���4��K+���������ϵ�K+������м�㻹��4�������Ҳ��4������12����������K+��8��$\frac{1}{8}$+6��$\frac{1}{2}$=4��CN-��12��$\frac{1}{4}$+1=4��K+��K+�ĺ˼��a�������ⳤΪ$\sqrt{2}$a�����������V=��$\sqrt{2}$a��3������������m=��•V��V=$\frac{m}{��}$=$\frac{{4M}_{��KCN��}}{{N}_{A}•��}$=$\frac{4��65}{{N}_{A}•��}$=��$\sqrt{2}$a��3����a=$\frac{\sqrt{2}}{2}��\root{3}{\frac{260}{��•{N}_{A}}}$��
�ʴ�Ϊ��12��$\frac{\sqrt{2}}{2}��\root{3}{\frac{260}{��•{N}_{A}}}$��
���� ���⿼�������Ӽ۵����Ų�����һ�����ܴ�С����ѧ����������ӻ����͡������ļ���ȣ����Ǹ߿���Ƶ���㣬�Ѷ����У�ע���̯���ڼ����е�Ӧ�ã�

| ��Ʒ�� | GB5461 |
| ��Ʒ�ȼ� | һ�� |
| �� �� | ʳ�Ρ�����ء������ |
| �⺬������I�ƣ� | 20��50mg/kg |
1IO3-+5I-+6H+=3I2+3H2O
��2��������Ӧ���ɵ�I2�������Ȼ�̼���飮�������Ȼ�̼��Һ�м���Na2SO3ϡ��Һ����I2��ԭ���Ի������Ȼ�̼��
��Na2SO3ϡ��Һ��I2��Ӧ�����ӷ���ʽ��I2+SO32-+H2O=2I-+SO42-+2H+��
��ijѧ����ƻ������Ȼ�̼�IJ�������Ϊ��
a����������Ȼ�̼��Һ���ڷ�Һ©���У�b����������Na2SO3ϡ��Һ��c��������²�Һ�壮
�����������©�IJ����������������е�λ���ڲ���b�����Ӳ�����
��ȱ����Ϊ����Һ©���������
��3����֪��I2+2S2O32-=2I-+S4O62-��ijѧ���ⶨʳ�þ����εĵ⺬�����䲽��Ϊ��
a��ȷ��ȡ12.7gʳ�Σ�����������ˮʹ����ȫ�ܽ⣻
b����ϡ�����ữ������Һ����������KI��Һ��ʹKIO3��KI��Ӧ��ȫ��
c���Ե���Ϊָʾ������μ������ʵ���Ũ��Ϊ6.0��10-4mol•L-1��Na2S2O3��Һ20.0mL��ǡ�÷�Ӧ��ȫ��
���ж�c�з�Ӧǡ����ȫ���ݵ������ǵ������һ����Һʱ����Һ����ɫǡ����ʧ���Ұ�����ڲ��ָ�ԭɫ����ﵽ�ζ��յ㣮
�ڸ�������ʵ��Ͱ�װ��˵�������⾫���εĵ⺬����20 mg/kg��
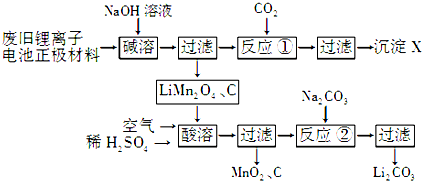
��1��X�Ļ�ѧʽΪAl��OH��3��
��2��д�������ܡ������з�Ӧ�����ӷ���ʽ��4LiMn2O4+O2+4H+�T4Li++8MnO2+2H2O��
��3����Ӧ������Na2CO3��Ӧ��������Li2SO4��H2SO4���ѧʽ������ҵ��ϴ��Li2CO3�õ�����ˮ��������ˮ����ԭ���ǽ���Li2CO3���ܽ�ȣ������ܽ⣮
��4�����෨�Ʊ�LiMn2O4��ʵ��������£���MnO2��Li2CO3��4��1�����ʵ���֮�����ϣ���ĥ3��5Сʱ��Ȼ�����£����¼��ȣ�����24Сʱ����ȴ�����£�
��д���÷�Ӧ�Ļ�ѧ����ʽ��8MnO2+2Li2CO3$\frac{\underline{\;����\;}}{\;}$4LiMn2O4+2CO2��+O2����
��LiMn2O4����Ԫ�ص�ƽ����̬Ϊ+3.5���ڲ�ͬ�¶��£��ϳɵ�LiMn2O4��Mn2+��Mn3+��Mn4+�ĺ������¶ȵĹ�ϵ���±���
| T/�� | w��Mn2+����%�� | w��Mn3+����%�� | w��Mn4+����%�� |
| 700 | 5.56 | 44.58 | 49.86 |
| 750 | 2.56 | 44.87 | 52.57 |
| 800 | 5.50 | 44.17 | 50.33 |
| 850 | 6.22 | 44.40 | 49.38 |
| A�� | ����ʳƷ���Ӽ������ʾ������彡���к� | |
| B�� | ĥ�����Ĵ��������ʣ�������к��ʱ���˰����� | |
| C�� | �ִ���սͨ�����Һ��SiCl4������ˮ�⣩��Һ���ɲ�����Ļ������Ҫ�ɷ���NH4Cl | |
| D�� | ����ˮ��ʱ�������������ԵĽ������ӣ�����Ư�� |
| A�� | ���ʯ��ʯī��Ϊͬ�������� | B�� | H��D��T��Ϊͬλ�� | ||
| C�� | CH3-CH3��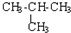 ��Ϊͬϵ�� ��Ϊͬϵ�� | D�� | 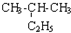 �� ��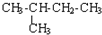 ��Ϊͬ���칹�� ��Ϊͬ���칹�� |
| A�� | a��������b������ | |
| B�� | �ŵ�����У�a��b�缫�Ͼ�����������ԭ��Ӧ | |
| C�� | �õ�ع���ʱ���Ӽ״���һ����������Һ�� pH ���ͣ���Ӧ�����ӷ���ʽΪ��CH3OH+8OH--6e-�TCO32-+6H2O | |
| D�� | ���ô˵�Դ��ⱥ��ʳ��ˮ��ÿ���� 0.1molO2���������������� 0.2molCl2�� |

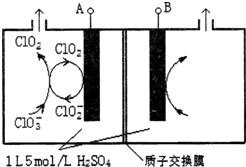 �������ȣ�ClO2���ǹ�����ϵĸ�Ч�����ס����١���ȫ����ɱ��������������Ϊ����4��������������ҵ�Ͽɲ��������ƣ�NaClO3�����������ƣ�NaClO2��Ϊԭ���Ʊ�ClO2��
�������ȣ�ClO2���ǹ�����ϵĸ�Ч�����ס����١���ȫ����ɱ��������������Ϊ����4��������������ҵ�Ͽɲ��������ƣ�NaClO3�����������ƣ�NaClO2��Ϊԭ���Ʊ�ClO2��