��Ŀ����
ϩ��A��һ�������¿�������Ŀ�ͼ���з�Ӧ��
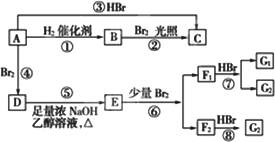
F1��F2��Ϊͬ���칹�壬G1��G2��Ϊͬ���칹�壬D��CCH3BrH3CCCH3BrCH3��
��֪1��3������ϩ��������ˮ�ӳ�ʱ���������ַ�ʽ��
CH2CHCHCH2��Br2��CH2BrCHBrCHCH2(1��2���ӳ�)
CH2CHCHCH2��Br2��CH2BrCHCHCH2Br(1��4���ӳ�)
�Իش��������⣺
(1)A�Ľṹ��ʽΪ________��
(2)��ͼ������ȡ����Ӧ����(�����ִ���)________��
(3)��ͼ�Т١��ۡ�������________��Ӧ��
(4)G1�Ľṹ��ʽΪ________��

��9�֣���1���±�Ϊϩ��������巢���ӳɷ�Ӧ��������ʣ�����ϩΪ������
| ϩ����� | ������� |
| (CH3)2C=CHCH3 | 10.4 |
| CH3CH=CH2 | 2.03 |
| CH2=CH2 | 1.00 |
| CH2=CHBr | 0.04 |
A��(CH3)2C=C(CH3)2 B��CH3CH=CHCH2CH3 C��CH2="CH" CH3 D��CH2=CHBr
��2��0.5molijȲ���������1molHCl�����ӳɷ�Ӧ�õ��ȴ��������ɵ��ȴ����������3mol Cl2����ȡ����Ӧ������ֻ��C��Cl����Ԫ�صĻ����������Ľṹ��ʽ�� ��
��3��ij������A������Է�������Ϊ104��̼����������Ϊ92.3����
��A�����п��ܹ�ƽ���̼ԭ������� ����
�ڷ�����A��һ�������¿����ɼӾ۸߷��ӣ��ø߷��ӽṹ�е�����Ϊ ��
��һ�������£�A��������Ӧ���õ��Ļ�������̼����������Ϊ85.7����д���γɸû�������л���Ӧ����ʽ ��
����֪
 ����д��A��ϡ�����KMnO4��Һ�ڼ��������·�Ӧ������Ľṹ��ʽ ��
����д��A��ϡ�����KMnO4��Һ�ڼ��������·�Ӧ������Ľṹ��ʽ �� ��9�֣���1���±�Ϊϩ��������巢���ӳɷ�Ӧ��������ʣ�����ϩΪ������
|
ϩ����� |
������� |
|
(CH3)2C=CHCH3 |
10.4 |
|
CH3CH=CH2 |
2.03 |
|
CH2=CH2 |
1.00 |
|
CH2=CHBr |
0.04 |
���л���������ӳ�ʱ��ȡ���������ʵ�Ӱ������й������ƣ����з�Ӧ����������_______________������ţ���
A��(CH3)2C=C(CH3)2 B��CH3CH=CHCH2CH3 C��CH2=CH CH3 D��CH2=CHBr
��2��0.5molijȲ���������1molHCl�����ӳɷ�Ӧ�õ��ȴ��������ɵ��ȴ����������3mol Cl2����ȡ����Ӧ������ֻ��C��Cl����Ԫ�صĻ����������Ľṹ��ʽ�� ��
��3��ij������A������Է�������Ϊ104��̼����������Ϊ92.3����
��A�����п��ܹ�ƽ���̼ԭ������� ����
�ڷ�����A��һ�������¿����ɼӾ۸߷��ӣ��ø߷��ӽṹ�е�����Ϊ ��
��һ�������£�A��������Ӧ���õ��Ļ�������̼����������Ϊ85.7����д���γɸû�������л���Ӧ����ʽ ��
����֪ ����д��A��ϡ�����KMnO4��Һ�ڼ��������·�Ӧ������Ľṹ��ʽ ��
����д��A��ϡ�����KMnO4��Һ�ڼ��������·�Ӧ������Ľṹ��ʽ ��


 ����д��A��ϡ�����KMnO4��Һ�ڼ��������·�Ӧ������Ľṹ��ʽ
����д��A��ϡ�����KMnO4��Һ�ڼ��������·�Ӧ������Ľṹ��ʽ

 ��F������ֻ��������ԭ�ӣ����ҽ��ڲ����ڵ�̼ԭ���ϣ�
��F������ֻ��������ԭ�ӣ����ҽ��ڲ����ڵ�̼ԭ���ϣ�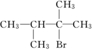


 ��F������ֻ��������ԭ�ӣ����ҽ��ڲ����ڵ�̼ԭ���ϡ�
��F������ֻ��������ԭ�ӣ����ҽ��ڲ����ڵ�̼ԭ���ϡ�