��Ŀ����
14�� ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺��1����Ũ��������ʵ���Ũ��Ϊ12mol/L��
��2��ijѧ����������Ũ���������ˮ����250mL���ʵ���Ũ��Ϊ0.7mol/Lϡ���ᣮ
�ٸ�ѧ������Ͳ��ȡ14.6 mL����Ũ����������ƣ�
�������ʵ�������У��ٽ�ͷ�ιܡ����ձ�������Ͳ���ܲ�����������ϡ����ʱ����ȱ�ٵ�������250mL����ƿ��
�����в������������Ƶ�ϡ��������ʵ���Ũ��ƫ�͵���A��D��E ������ĸ����
A������Ͳ��ȡŨ����ʱ���Ӱ�Һ��
B��δ�ָ������¾ͽ���Һע������ƿ�����ж���
C������ƿ������ˮϴ��δ����
D������ʱ����Һ��
E��δϴ���ձ��Ͳ�����
��3�����ڱ�״���£���a L HCl��������1Lˮ�У�������Һ�ܶ�Ϊd g/mL�������Һ�����ʵ���Ũ��Ϊdmol/L��
a��$\frac{36.5a}{22.4��a+1��d}$ b��$\frac{1000ad}{36.5a+22.4}$ c��$\frac{ad}{36.5a+22400}$ d��$\frac{1000ad}{36.5a+22400}$��
���� ��1���������ʵ���Ũ��c=$\frac{1000�Ѧ�}{M}$�����㣻
��2���ٸ�����Һϡ�Ͷ���CŨVŨ=CϡVϡ�����㣻
�ڸ������Ʋ����Ǽ��㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ��������
�۸���c=$\frac{n}{V}$��������ʵ����ʵ���n����Һ�����V�ı仯��������������
��3������n=$\frac{V}{{V}_{m}}$�����Ȼ�����������ʵ������ٸ���m=nM����HCl������������m=��V����ˮ������������������Һ������������V=$\frac{m}{��}$������Һ�����������c=$\frac{n}{V}$�������������ʵ���Ũ�ȣ�
��� �⣺��1����Һ�����ʵ���Ũ��c=$\frac{1000�Ѧ�}{M}$=$\frac{1000��1.2��36.5%}{36.5}$=12mol/L���ʴ�Ϊ��12��
��2�����������Ũ��������ΪVmL��������Һϡ�Ͷ���CŨVŨ=CϡVϡ��֪��12mol/L��VmL=0.7mol/L��250mL�����V=14.6mL���ʴ�Ϊ��14.6��
�ڸ������Ʋ����Ǽ��㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��֪�������������Ͳ���ձ�����������250mL����ƿ�ͽ�ͷ�ιܣ��ʻ�ȱ��250mL����ƿ���ʴ�Ϊ��250mL����ƿ��
��A������Ͳ��ȡŨ����ʱ���Ӱ�Һ�棬��Ũ��������ƫС�����Ƴ�����Һ��Ũ��ƫС����A��ȷ��
B��δ�ָ������¾ͽ���Һע������ƿ�����ж��ݣ�����ȴ����Һ���ƫС��Ũ��ƫ�ߣ���B����
C������ƿ������ˮϴ��δ�����������Һ��Ũ����Ӱ�죬��C����
D������ʱ����Һ�棬����Һ���ƫ��Ũ��ƫС����D��ȷ��
E��δϴ���ձ��Ͳ�������������ʵ���ʧ����Ũ��ƫС����Eѡ��
��ѡADE��
��3��HCl�����ʵ���Ϊ$\frac{aL}{22.4L/mol}$=$\frac{a}{22.4}$mol��HCl������Ϊ$\frac{a}{22.4}$mol��36.5g/mol=$\frac{36.5a}{22.4}$g��1Lˮ������Ϊ1000mL��1g/mL=1000g������Һ������Ϊ��$\frac{36.5a}{22.4}$+1000��g����Һ�����Ϊ$\frac{��\frac{36.5a}{22.4}+1000��g}{1000dg/L}$=$\frac{36.5a+22400}{22400d}$L����������Һ�����ʵ���Ũ��Ϊ$\frac{\frac{a}{22.4}L}{\frac{36.5a+22400}{22400d}L}$=$\frac{1000da}{36.5a+22400}$mol/L��
��ѡd��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ���������������ڻ�������Ŀ���ѶȲ���

| A�� | ����ı�ȼ����Ϊ-890.3 kJ•mol-1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪCH4��g��+2O2��g���TCO2��g��+2H2O��g����H=-890.3 kJ•mol-1 | |
| B�� | 500�桢300 MPa�£���0.5 mol N2��1.5 mol H2�����ܱ������г�ַ�Ӧ����NH3��g��������19.3 kJ�����Ȼ�ѧ����ʽΪN2��g��+3H2��g��$?_{500�桢300MPa}^{����}$ 2NH3��g����H=-38.6kJ•mol-1 | |
| C�� | �Ȼ�þ��Һ�백ˮ��Ӧ��Mg2++2OH-�TMg��OH��2�� | |
| D�� | ����������NaOH��Һ��A12O3+2OH-�T2AlO${\;}_{2}^{-}$+H2O |
| A�� | ̼���� | B�� | Һ̬�� | C�� | ���� | D�� | �������� |

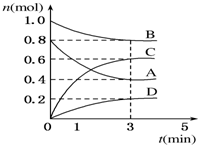 T��ʱ�����ݻ�Ϊ0.5L���ܱ������з����� �·�Ӧ��mA��g��+nB��g��?pC��g��+qD��s����H��0��m��n��p��qΪ��������ȣ���A��B��C��D�����ʵ����仯��ͼ��ʾ��
T��ʱ�����ݻ�Ϊ0.5L���ܱ������з����� �·�Ӧ��mA��g��+nB��g��?pC��g��+qD��s����H��0��m��n��p��qΪ��������ȣ���A��B��C��D�����ʵ����仯��ͼ��ʾ��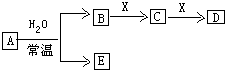 �ɶ�����Ԫ����ɵ���ѧ��������A��B��C��D��E��X������ͼת����ϵ������������ͷ�Ӧ������ȥ����
�ɶ�����Ԫ����ɵ���ѧ��������A��B��C��D��E��X������ͼת����ϵ������������ͷ�Ӧ������ȥ���� ��
�� ��
��