��Ŀ����
(16��)�����������壨FeSO4��7H2O����ҽҩ������Ѫ����ij����С������KMnO4��Һ�ζ��ķ������ⶨ�ò�Ѫ������Ԫ�صĺ���������������ʵ�飺
[��������]
�����������£���KMnO4����Һ����������ԭ�ζ������ԲⶨFe2+�ĺ�������Ӧ�����ӷ���ʽ�ǣ�Fe2++MnO4-+H+����Fe3++Mn2++H2O��δ��ƽ��
[��ʵ����Ʒ]
��������a.������ƽ��b.�ζ��ܣ�c.100mL��Ͳ��d.�ձ���e.©����f.����ƿ��100 mL�� 250mL����g.��ƿ��h.��������i.ҩ�ף�j.��ƿ��k.����̨�����ζ��ܼУ���l.��ͷ�ιܡ�
 [ʵ���¼]
[ʵ���¼]
[����������]
��1������ʵ����Ʒ�У�һ������Ҫ�������У�����ţ� ������Ҫ���Լ��У�����ţ� ��
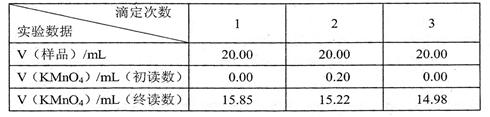
�� 2����ʵ�����õ�KMnO4����Һ�����ʵ���Ũ��Ϊ
2����ʵ�����õ�KMnO4����Һ�����ʵ���Ũ��Ϊ  ��
��
��3������С������λͬѧ������������ֵζ���ʽ���гֲ�����ȥ����������ͬѧ�ǵ����ۣ����ȡ�ù�ʶ����Ϊ��������� ������ĸ��ţ���
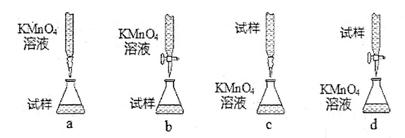
��4���жϵζ��յ�������� ��
��5�����ڽӽ��ζ��յ�ʱ������������ˮ����ƿ�ڱڳ� ϴһ�£��ټ����ζ����յ㣬������õIJ�Ѫ������Ԫ�صĺ��� ��ƫ��ƫС����Ӱ�죩��
ϴһ�£��ټ����ζ����յ㣬������õIJ�Ѫ������Ԫ�صĺ��� ��ƫ��ƫС����Ӱ�죩��
��6������ʵ�����ݣ�����ò�Ѫ������Ԫ�ص��������� ��

[��������]
�����������£���KMnO4����Һ����������ԭ�ζ������ԲⶨFe2+�ĺ�������Ӧ�����ӷ���ʽ�ǣ�Fe2++MnO4-+H+����Fe3++Mn2++H2O��δ��ƽ��
[��ʵ����Ʒ]
��������a.������ƽ��b.�ζ��ܣ�c.100mL��Ͳ��d.�ձ���e.©����f.����ƿ��100 mL�� 250mL����g.��ƿ��h.��������i.ҩ�ף�j.��ƿ��k.����̨�����ζ��ܼУ���l.��ͷ�ιܡ�
 [ʵ���¼]
[ʵ���¼][����������]
��1������ʵ����Ʒ�У�һ������Ҫ�������У�����ţ� ������Ҫ���Լ��У�����ţ� ��
��
 2����ʵ�����õ�KMnO4����Һ�����ʵ���Ũ��Ϊ
2����ʵ�����õ�KMnO4����Һ�����ʵ���Ũ��Ϊ  ��
����3������С������λͬѧ������������ֵζ���ʽ���гֲ�����ȥ����������ͬѧ�ǵ����ۣ����ȡ�ù�ʶ����Ϊ��������� ������ĸ��ţ���

��4���жϵζ��յ�������� ��
��5�����ڽӽ��ζ��յ�ʱ������������ˮ����ƿ�ڱڳ�
 ϴһ�£��ټ����ζ����յ㣬������õIJ�Ѫ������Ԫ�صĺ��� ��ƫ��ƫС����Ӱ�죩��
ϴһ�£��ټ����ζ����յ㣬������õIJ�Ѫ������Ԫ�صĺ��� ��ƫ��ƫС����Ӱ�죩����6������ʵ�����ݣ�����ò�Ѫ������Ԫ�ص��������� ��
(16��)
�� e j ��2�� �� c d f��2�֣�
�� c d f��2�֣�
��1.200��10-2mol/L ��3�֣���Ч���ֲ���Ҫ�� ��b��2�֣�
�ȵ������һ��KMnO4��Һ����Һǡ������ɫ��dz�Ϻ�ɫ���Ұ�����ڲ���ɫ��2�֣�
����Ӱ�죨2�֣�
��16.8%��3�֣�
�� e j ��2��
 �� c d f��2�֣�
�� c d f��2�֣���1.200��10-2mol/L ��3�֣���Ч���ֲ���Ҫ�� ��b��2�֣�
�ȵ������һ��KMnO4��Һ����Һǡ������ɫ��dz�Ϻ�ɫ���Ұ�����ڲ���ɫ��2�֣�
����Ӱ�죨2�֣�
��16.8%��3�֣�
��

��ϰ��ϵ�д�
 ����ʦ���һ��һ��ϵ�д�
����ʦ���һ��һ��ϵ�д�
�����Ŀ