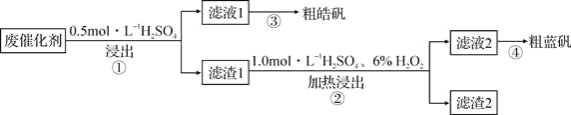
��Ŀ����
����Ŀ����Ӧ2C+O2=2CO�������仯��ͼ��ʾ������˵����ȷ���ǣ� ��

A.12gC(s)��һ����O2(g)��Ӧ����14gCO(g)�ų�������Ϊ110.5kJ
B.2molC(s)������O2(g)��Ӧ����CO2(g)�ų�����������221kJ
C.�÷�Ӧ���Ȼ�ѧ����ʽ��2C(s)+O2(g)=2CO(g) ��H=-221kJ
D.�÷�Ӧ�ķ�Ӧ�ȵ������ɵ�CO(g)�������е�������μӷ�Ӧ��O2(g)�������е������IJ�
���𰸡�B
��������
��ͼ����Ϣ���Կ�����C(s)+![]() O2(g)=CO(g) ��H=-110.5kJ/mol��
O2(g)=CO(g) ��H=-110.5kJ/mol��
A����ͼ����Ϣ��֪��C(s)��һ����O2(g)��Ӧ����14gCO(g)�ų�������Ϊ55.25kJ��A����ȷ��
B��2molC(s)������O2(g)��Ӧ����CO (g)ʱ������110.5kJ��������CO2ʱ���ȴ���221kJ��B��ȷ��
C���÷�Ӧ���Ȼ�ѧ����ʽ��2C(s)+O2(g)=2CO(g) ��H=-221kJ/mol��C����ȷ��
D���÷�Ӧ�ķ�Ӧ�ȵ������ɵ�CO(g)�����е�������μӷ�Ӧ��C(s)��O2(g)�����е������D����ȷ��
��ѡB��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��һ�������£����淴Ӧ2A(g)![]() B(g)+3C(g)����Ӧ����ƽ��״̬���ǣ� ��
B(g)+3C(g)����Ӧ����ƽ��״̬���ǣ� ��
ѡ�� | ����Ӧ���� | �淴Ӧ���� |
A | v(A)=2mol��L-1��min-1 | v(B)=2mol��L-1��min-1 |
B | v(A)=2mol��L-1��min-1 | v(C)=2mol��L-1��min-1 |
C | v(A)=1mol��L-1��min-1 | v(B)=2mol��L-1��min-1 |
D | v(A)=1mol��L-1��min-1 | v(C)=1.5mol��L-1��min-1 |
A.AB.BC.CD.D