��Ŀ����
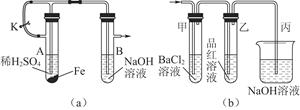
����ͭ��һ���/����ͭ����������Բ��ϣ���ͨ�����з����Ʊ����ڲ����н�����ͭ�ù�����40%������ܽ⣬���Ƴ�CuF2��5HF��5H2O���ٽ���������������ڵIJ����У��ڸ���ķ�������������400%���м�����ˮ�����ͨ�뵪����
��1���Ʊ��������ò�������ò��������ԭ����______________________________________���û�ѧ����ʽ��ʾ����
��2�� �/����ͭ��طŵ�ʱ�ܷ�ӦΪ�û���Ӧ���仯ѧ����ʽΪ____________________________________________________��
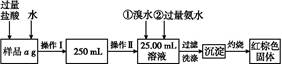
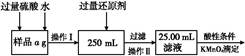
��3������ȡ�����Ʊ�����Ʒ������CuF2��CuO�� 2.120 g����ͨ����м���ϡ��������ȫ�ܽ⣬Ȼ���������������������Һ���ó�����������������գ���1.680 g��ɫ���壬������Ʒ��CuF2��CuO�����ʵ���֮�ȡ�
��1���Ʊ��������ò�������ò��������ԭ����______________________________________���û�ѧ����ʽ��ʾ����
��2�� �/����ͭ��طŵ�ʱ�ܷ�ӦΪ�û���Ӧ���仯ѧ����ʽΪ____________________________________________________��
��3������ȡ�����Ʊ�����Ʒ������CuF2��CuO�� 2.120 g����ͨ����м���ϡ��������ȫ�ܽ⣬Ȼ���������������������Һ���ó�����������������գ���1.680 g��ɫ���壬������Ʒ��CuF2��CuO�����ʵ���֮�ȡ�
��1��SiO2��4HF=SiF4����2H2O
��2��CuF2��2Li=Cu��2LiF
��3��20:1
��2��CuF2��2Li=Cu��2LiF
��3��20:1
��1�������ḯʴ����������SiF4����2�������Ϣ���Ѿ������˷�Ӧ����﮺ͷ���ͭ����ע���˷�Ӧ���͡���3��������֪��CuF2��CuO��CuSO4��Cu��OH��2��CuO���ʿ��ò��������CuF2��
CuF2����������CuO������m
�� 102 g 80 g 22 g
m��CuF2����������������2.120 g��1.680 g��0.440 g
m��CuF2���� ��2.040 g
��2.040 g
n��CuF2���� ��
�� ��0.020 mol
��0.020 mol
m��CuO����2.120 g��2.040 g��0.080 g
n��CuO���� ��
�� ��0.001 mol��
��0.001 mol�� ��
�� ��20��1
��20��1
CuF2����������CuO������m
�� 102 g 80 g 22 g
m��CuF2����������������2.120 g��1.680 g��0.440 g
m��CuF2����
 ��2.040 g
��2.040 gn��CuF2����
 ��
�� ��0.020 mol
��0.020 molm��CuO����2.120 g��2.040 g��0.080 g
n��CuO����
 ��
�� ��0.001 mol��
��0.001 mol�� ��
�� ��20��1
��20��1
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ

 E(SCN)3��
E(SCN)3��