��Ŀ����
��ʯ����Ҫ�ɷ�ΪCaCO3������ͼ�����ʵ��ת���У�K��I��J���ճ������еĵ�ζ����I��J��M��GΪ�л��Mr(J)=60��Mr(M)=88����Ӧ�٢ڢ۾��ǹ�ҵ�����е���Ҫ��Ӧ��
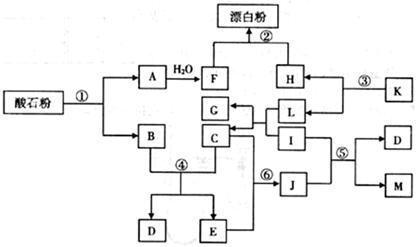
��ش��������⣺
��1��K�Ļ�ѧʽ�� ��J�ķ���ʽ�� ��
��2����Ӧ���У���10g CaCO3����ʯ�ۣ���![]() �桢
�桢![]() kPaʱ����ȫ�ֽ�����A(s)��B(g)������a kJ���������ʲ���Ӧ�����÷�Ӧ���Ȼ�ѧ����ʽΪ ��
kPaʱ����ȫ�ֽ�����A(s)��B(g)������a kJ���������ʲ���Ӧ�����÷�Ӧ���Ȼ�ѧ����ʽΪ ��
��3����Ӧ�ܵĻ�ѧƽ�ⳣ���ı���ʽ��K= ����֪![]() ��÷�Ӧ�� ��Ӧ������ȡ����ȡ�����
��÷�Ӧ�� ��Ӧ������ȡ����ȡ�����
��4����Ӧ�ݵĻ�ѧ����ʽΪ ��
��5����Ӧ������J��������ɫ��ѧԭ���ԭ���� ��
��1��NaCl C2H4O2 ����2�֣�
��2��CaCO3(s) ![]() CaO(s) + CO2(g) ��H=��10a kJ/mol ��2�֣���������д��һ�֣�
CaO(s) + CO2(g) ��H=��10a kJ/mol ��2�֣���������д��һ�֣�
��3��![]() ���� ����2�֣�
���� ����2�֣�
��4��![]() ��2�֣�
��2�֣�
��5��ԭ��������Ϊ100%��.��.��/Դ/�� ��2�֣�
����:

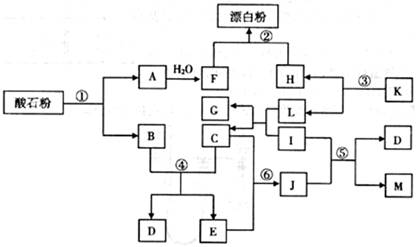
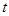 �桢
�桢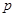 kPaʱ����ȫ�ֽ�����A(s)��B(g)������a kJ���������ʲ���Ӧ�����÷�Ӧ���Ȼ�ѧ����ʽΪ
��
kPaʱ����ȫ�ֽ�����A(s)��B(g)������a kJ���������ʲ���Ӧ�����÷�Ӧ���Ȼ�ѧ����ʽΪ
��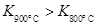 ��÷�Ӧ��
��Ӧ������ȡ����ȡ�����
��÷�Ӧ��
��Ӧ������ȡ����ȡ�����