��Ŀ����
20.�ڳ�ѹ��120��ʱ�����ܱ������г���H2S��O2�Ļ�����干100 mL���õ��ȼ������ַ�Ӧ�ָ���ԭ״�����ⶨ�����ڲ����������������ⶨ��������������V1��ԭ���������O2�����V2(O2)���Ӷ��仯�����ϵ����ͼ��ʾ��
 ������������
������������

(1)��A��B��C������й����������±���
|
|
A |
B |
C |
|
��Ӧǰ���������ɷ���� |
|
|
|
|
��Ӧ�����������ɷ���� |
|
|
|
(2)����V1��V2(O2)�Ĺ�ϵ�����ú�V1��V2(O2)�ĺ���ʽ��ʾ֮��
(3)�������������V1=90mL��ԭ�������ijɷ���________��________��
������
(1)
(2)��V2 (3)H2S��90mL��O2��10mL��H2S��20mL��O2��80mL
|

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д� ������������
������������

(1)��A��B��C������й����������±���
|
|
A |
B |
C |
|
��Ӧǰ���������ɷ���� |
|
|
|
|
��Ӧ�����������ɷ���� |
|
|
|
(2)����V1��V2(O2)�Ĺ�ϵ�����ú�V1��V2(O2)�ĺ���ʽ��ʾ֮��
(3)�������������V1=90mL��ԭ�������ijɷ���________��________��
��14�֣�ˮú����һ�ָ�Ч����ȼ�ϣ�����Ҫ�ɷ���CO��H2������ˮ����ͨ�����ȵ�̿�Ƶã�C (s) + H2O(g) CO (g) +H2 (g) ��H��+131kJ?mol��1
CO (g) +H2 (g) ��H��+131kJ?mol��1
��T�¶��£��ĸ������о�������������Ӧ����������̿�������������ʵ����ʵ���Ũ�ȼ����淴Ӧ���ʹ�ϵ���±���ʾ������д������Ӧ�Ŀո�
| ���� ��� | c(H2O) /mol��L��1 | c(CO) /mol��L��1 | c(H2) /mol��L��1 | v����v���Ƚ� |
| I | 0.06 | 0.60 | 0.10 | v����v�� |
| II | 0.06 | 0.50 | 0.40 | �� |
| III | 0.12 | 0.40 | 0.80 | v����v�� |
| IV | 0.12 | 0.30 | �� | v����v�� |
������һ���ݻ��ɱ���ܱ����������º�ѹ�£������м���1.0mol̿��1.0 molˮ���� (H2O)������������Ӧ���ﵽƽ��ʱ�������������Ϊԭ����1.25 ����ƽ��ʱˮ������ת����Ϊ ����������в���a mol ̿��ˮ������ת���ʽ� ���� ����������С���������䡱����
����һ����������ˮú���ܺϳɼ״���CO(g)+2H2(g)
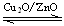 CH3OH(g) ��H��0���ϳɼ״���Ӧ��ϵ��ͨ������CO��ƽ���� �ƶ�����Сѹǿ��ƽ���� �ƶ��������¶���ƽ���� �ƶ��������ң�����
CH3OH(g) ��H��0���ϳɼ״���Ӧ��ϵ��ͨ������CO��ƽ���� �ƶ�����Сѹǿ��ƽ���� �ƶ��������¶���ƽ���� �ƶ��������ң�������������ȼ�ϵ�ؾ��иߵķ���Ч�ʣ��õ����Li2CO3��Na2CO3�������λ����������ʣ�����һ��ͨ��CO����һ��ͨ�������CO2�Ļ�����塣����������Ϣ����ȼ�ϵ�أ�������ӦʽΪ ��������ӦʽΪ ��
��5����֪�ڳ��³�ѹ�£�
��2CH3OH (l)��3O2 (g) == 2CO2 (g)��4H2O (g)����H1����1 275.6 kJ��mol��1
��2CO (g)��O2 (g) == 2CO2 (g) ��H2����566.0 kJ��mol��1
��H2O (g) = H2O (l)����H3����44.0 kJ��mol��1
д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��____________________

