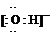��Ŀ����
���ܱ������У�����һ���¶Ƚ������·�Ӧ��N2(g)��3H2(g)![]() 2NH3(g)����֪����1 mol N2��3 mol H2���ں�ѹ�����´ﵽƽ��ʱ����a mol NH3���ں��������´ﵽƽ��ʱ����b mol NH3(���±��б�Ţٵ�һ��)������ͬ�����£��ﵽƽ��ʱ������и���ֵİٷֺ������䣮�ش��������⣺
2NH3(g)����֪����1 mol N2��3 mol H2���ں�ѹ�����´ﵽƽ��ʱ����a mol NH3���ں��������´ﵽƽ��ʱ����b mol NH3(���±��б�Ţٵ�һ��)������ͬ�����£��ﵽƽ��ʱ������и���ֵİٷֺ������䣮�ش��������⣺
(1)д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��K��________��
(2)ƽ�ⳣ��KֵԽ��������ƽ��ʱ________(�����)��
A��N2��ת����Խ��
B��NH3�IJ���Խ��
C������Ӧ���е�Խ����
D����ѧ��Ӧ�ٶ�Խ��
(3)a��b�Ĺ�ϵ�ǣ�a________b(�����������������)��
(4)������

�𰸣�
������

������
![]()
(4)


��ϰ��ϵ�д�
 һ���㶨ϵ�д�
һ���㶨ϵ�д� ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д� ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
�����Ŀ
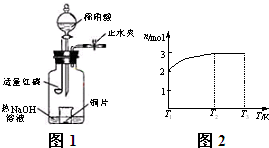 X��Y��Z��WΪ������ͬ�������ķ��ӻ����ӣ�����ԭ������С��10��Ԫ����ɣ�X��5��ԭ�Ӻˣ�ͨ��״���£�WΪ��ɫҺ�壮��֪��X+Y
X��Y��Z��WΪ������ͬ�������ķ��ӻ����ӣ�����ԭ������С��10��Ԫ����ɣ�X��5��ԭ�Ӻˣ�ͨ��״���£�WΪ��ɫҺ�壮��֪��X+Y