��Ŀ����
��ͼΪʵ����ijŨ�������Լ�ƿ��ǩ�ϵ��й����ݣ�
|
��1����Ũ��������ʵ���Ũ��Ϊ ��
��2��ʹ������ƿǰ������е�һ��������___________
��3��ijѧ����������Ũ���������ˮ����500 ml ���ʵ���
Ũ��Ϊ1.0 mol��L-1ϡ���ᡣ
�ٸ�ѧ����Ҫ��ȡ ml ����Ũ����������ơ�
������ƿ����������������е�
A�¶� BŨ�� C���� Dѹǿ E�̶���
�������ƹ����У�����ʵ������������Ƶ�ϡ��������ʵ���Ũ���к�Ӱ�죿
���ں�������ϡ�ƫ�ߡ�����ƫ�͡�������Ӱ�족����
I������Ͳ��ȡŨ����ʱ���Ӱ�Һ����������
II�����ݺ���ҡ�ȡ����ã�����Һ���½����ټ�����������ˮ������������
����ʱ�����ӿ̶���
����ת��ʱ����ƿ������������ˮ
��1��18.4 mol��L-1 (2) ����Ƿ�©ˮ
��3���� 27.2 �� ACE �� ƫ�� ƫ�� ƫ�� ��Ӱ��

 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д� Сѧ��ѧ������ѿڶ���ϵ�д�
Сѧ��ѧ������ѿڶ���ϵ�д� ������Ӧ�������������ϵ�д�
������Ӧ�������������ϵ�д� �㽭֮�ǿ�ʱ�Ż���ҵϵ�д�
�㽭֮�ǿ�ʱ�Ż���ҵϵ�д�ʵ���ҳ���Ũ������Ҵ���ϼ�����ȡ��ϩ��
��1��ʵ��������ϩ�Ļ�ѧ����ʽΪ ��
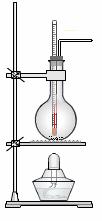 ��2��ʵ������Ũ������Ҵ���ϼ�������ϩ��������ͼ��ʾװ��,����˵����ȷ���� ��
��2��ʵ������Ũ������Ҵ���ϼ�������ϩ��������ͼ��ʾװ��,����˵����ȷ���� ��
A��Ũ����ֻ������
B���ڷ�Ӧ�����з��뼸Ƭ���Ƭ��ֹ���Һ����
C����Ӧ�¶Ȼ���������170��
D������ˮ���������������ռ���ϩ
E������ƿ��װ����4mL�Ҵ���12mL3mol/L H2SO4���Һ
F���¶ȼ�Ӧ���뷴Ӧ��ҺҺ���£��Ա�����¶�
G����Ӧ��Ϻ���Ϩ��ƾ��ƣ��ٴ�ˮ��ȡ������
��3��������װ���е��¶ȼƻ��ɷ�Һ©����������ȡ�������У��ƾ��ƿ��ÿɲ��ã� ��
A��CO2 B��NH3
C��O2 D��SO2
E��NO2 F��Cl2
��4�� ���¶ȹ��ߣ���Ӧ����Һ��ɫ�� ��ijͬѧ�������ʵ����ȷ��������������к�����ϩ��SO2��
|
|
��I��II��III��IVװ�ÿ�ʢ�ŵ��Լ��ǣ��뽫�����й��Լ����������ո��ڣ���
A��Ʒ�� B��NaOH��Һ C��Ũ���� D������KMnO4��Һ
I ��II ��III ��IV ��
����˵��SO2������ڵ������� ��
��ʹ��װ��II��Ŀ���� ��
��ʹ����III��Ŀ���� ��
��ȷ��������ϩ�������� ��
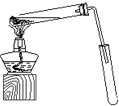 ij��ѧʵ��С������ͼ��ʾ��װ����ȡ������������������ �� �� �����Ƿ����������ʣ�����̨�����ӵ�֧������ʡ�ԣ�����֪���������ķе�Ϊ77.1�棬�Ҵ��е�Ϊ78.4�棬����ķе�Ϊ118�森�����Ҫ����գ�
ij��ѧʵ��С������ͼ��ʾ��װ����ȡ������������������ �� �� �����Ƿ����������ʣ�����̨�����ӵ�֧������ʡ�ԣ�����֪���������ķе�Ϊ77.1�棬�Ҵ��е�Ϊ78.4�棬����ķе�Ϊ118�森�����Ҫ����գ�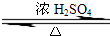 CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O ijͬѧΪ��ȡ�������������Թ�A�м���3mL�Ҵ���Ȼ������Թܱ���������
ijͬѧΪ��ȡ�������������Թ�A�м���3mL�Ҵ���Ȼ������Թܱ���������

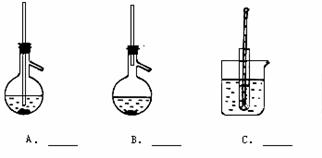
 ��4����ͼ��ʵ����������������װ��ͼ���ش��������⡣
��4����ͼ��ʵ����������������װ��ͼ���ش��������⡣