��Ŀ����
(10��)�ס��ҡ�����������������ˮ�����ʣ��ֱ���NH4+��Ba2+��Mg2+��H+��OH����Cl����HCO3����SO42���еIJ�ͬ�����Ӻ������Ӹ�һ����ɡ���֪��
�ٽ�����Һ�ֱ��������������ʵ���Һ��ϣ����а�ɫ�������ɣ�
��0.1mol/L����Һ��c(H+)>0.1mol/L��
�������Һ�е���AgNO3��Һ�в�����ϡHNO3�İ�ɫ�������ɡ��ش�
��1������Һ��
��2������Һ�ĵ��뷽��ʽ
��3������ҺPH 7, ԭ���÷���ʽ��ʾ
��4���ҺͶ���Ӧ�����ӷ���ʽ
��1��NH4HCO3 ��2��H2SO4===2H+ +SO42�� ��3�� �� Mg2++2H2O === Mg(OH)2 +2H+
��4��H+ + HCO3��=== CO2��+H2O
��������
������������ݢ��е���Ϣ��֪���Ƕ�Ԫ�ᣬ������H2SO4�����ݢ�����������֪���к���Cl-���ٽ�Ϣ����ṩ��Ϣ�����������������ʻ�Ͼ�������ɫ����������Ƴ�����Ba��OH��2������H2SO4������MgCl2������NH4HCO3��(1) ����Һ��NH4HCO3��(2) ������ǿ����ʣ�����뷽��ʽ�ǣ�H2SO4===2H+ +SO42����(3) ����ҺPH��7, ԭ����������þ���������Һ�п���ˮ�⣬�����ӷ���ʽ��ʾΪ��Mg2++2H2O === Mg(OH)2 +2H+��(4) �ҺͶ���Ӧ�����ӷ���ʽΪ��H++HCO3��===CO2��+H2O��
���㣺�������ӵļ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���15�֣�Ϊ��̽��H2O2��H2SO3��Br2�����Ե����ǿ�����������ʵ�飨�г���������ȥ������ش��������⣺

��1������A������_________����������___________��
��2��������B�μ�Һ�岢����Ҫ������c��ԭ����____________________________��
��3��ʵ���¼���£��벹ȫ�հף���
���� | ʵ����� | ʵ������ | ʵ����� |
�� | ����a����μ���H2SO3��Һ������ | ________________ | __________________________ |
�� | �����������Һ����μ���H2O2��Һ | �տ�ʼ��Һ��ɫ�����Ա仯�������μӣ���Һ��Ϊ�Ȼ�ɫ | __________________________ |
��4��������У���ʼʱ��ɫ�����Ա仯��ԭ���ǣ�д��һ����_______________________��
������з�Ӧ�����ӷ���ʽ_________________________________________________��
���������Ҫ��Ӧ�����ӷ���ʽ_____________________________________________��
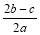 B��
B��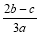 C��
C�� 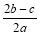 D��
D��

 mol��L��1
mol��L��1