��Ŀ����
ij��������������������������Ư�ۣ��ó�������Ʒ˵�������£�
��1��Ư�۵���Ч�ɷ���
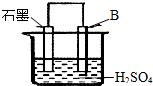
��2������������Cl2��Դ�ڵ��ʳ��ˮ���������У�������ӦΪ2Cl--2e-=Cl2����������ӦΪ2H2O+2e-=2OH-+H2��������ʱ��������
��3������Ư��ԭ���ǣ��û�ѧ����ʽ��ʾ��
��4�����ܷ�ܹⱣ�������������������䡱����ΪƯ����Ч�ɷ��������CO2��H2O��Ӧ���ɲ��ȶ��Ĵ��������ʧЧ�����ɴ�����Ļ�ѧ����ʽΪ
��5��ij�������У�ȡһ������Ư���ܽ���1000gˮ�У�������Һ������ƺ��Ȼ��Ƶ����ʵ���Ũ�Ⱦ�Ϊ0.01mol/L������Ư�۲����������ʣ���������Һ�������Ϊ1L����˴���Һ�����У�����Ư�۵�����Ϊ
��������1��Ư�۵���Ҫ�ɷ��Ǵ�����ƺ��Ȼ��ƣ���Ч�ɷ��Ǵ�����ƣ�
��2�����NaCl��Һ����������������Ӧ���������������ŵ���������������������ԭ��Ӧ�������ӷŵ�����������
��3����ҵ��ʯ�����������Ӧ�Ʊ�Ư�ۣ�
��4��Ư���к���Ca��ClO��2����������е�ˮ�Ͷ�����̼��Ӧ���ɲ��ȶ���HClO�����ʣ�
��5��Ư�۲����������ʣ��ɷ�Ϊ������ƺ��Ȼ��ƣ�������Ŀ�����ṩ�����ݽ�� m=n��M���
��2�����NaCl��Һ����������������Ӧ���������������ŵ���������������������ԭ��Ӧ�������ӷŵ�����������
��3����ҵ��ʯ�����������Ӧ�Ʊ�Ư�ۣ�
��4��Ư���к���Ca��ClO��2����������е�ˮ�Ͷ�����̼��Ӧ���ɲ��ȶ���HClO�����ʣ�
��5��Ư�۲����������ʣ��ɷ�Ϊ������ƺ��Ȼ��ƣ�������Ŀ�����ṩ�����ݽ�� m=n��M���
����⣺��1�����ڴ���������Ա�̼����������������������̼��ˮ��Ӧ������Ư�۾���Ư���Ե�ԭ���ǣ�Ca��ClO��2+CO2+H2O=CaCO3+2HClO��HClO����Ư���ԣ�����Ч�ɷ�ΪCa��ClO��2���ʴ�Ϊ��Ca��ClO��2��
��2����������ԭ��Ӧ�У����ϼ����ߵķ�ӦΪ������Ӧ�����ʳ��ˮ������ӦΪ2Cl--2e-=Cl2����������������������Ӧ���ʴ�Ϊ��������
��3����ҵ��ʯ�����������Ӧ�Ʊ�Ư�ۣ���Ӧ�ķ���ʽΪ2Ca��OH��2+2Cl2�TCaCl2+Ca��ClO��2+2H2O���ʴ�Ϊ��2Ca��OH��2+2Cl2�TCaCl2+Ca��ClO��2+2H2O��
��4��Ư���к���Ca��ClO��2����������е�ˮ�Ͷ�����̼��Ӧ���ɲ��ȶ���HClO����Ӧ�ķ���ʽΪCa��ClO��2+CO2+H2O�TCaCO3��+2HClO������������ֽ�������������������ʣ��ʴ�Ϊ��Ca��ClO��2+CO2+H2O�TCaCO3��+2HClO��
��5��������ƺ��Ȼ��Ƶ����ʵ���Ũ�Ⱦ�Ϊ0.01mol/L��������Һ�������Ϊ1L������Һ�к��д�����ƺ��Ȼ��Ƶ����ʵ�����Ϊ0.01mol������m=n��M�������������Ϊm=n��M=0.01mol��143g/mol=1.43g���Ȼ��Ƶ�����Ϊm=n��M=0.01mol��111g/mol=1.11g��Ư�۲����������ʣ��ɷ�Ϊ������ƺ��Ȼ��ƣ�����Ư�۵�����Ϊ2.54g��
�ʴ�Ϊ��2.54g��
��2����������ԭ��Ӧ�У����ϼ����ߵķ�ӦΪ������Ӧ�����ʳ��ˮ������ӦΪ2Cl--2e-=Cl2����������������������Ӧ���ʴ�Ϊ��������
��3����ҵ��ʯ�����������Ӧ�Ʊ�Ư�ۣ���Ӧ�ķ���ʽΪ2Ca��OH��2+2Cl2�TCaCl2+Ca��ClO��2+2H2O���ʴ�Ϊ��2Ca��OH��2+2Cl2�TCaCl2+Ca��ClO��2+2H2O��
��4��Ư���к���Ca��ClO��2����������е�ˮ�Ͷ�����̼��Ӧ���ɲ��ȶ���HClO����Ӧ�ķ���ʽΪCa��ClO��2+CO2+H2O�TCaCO3��+2HClO������������ֽ�������������������ʣ��ʴ�Ϊ��Ca��ClO��2+CO2+H2O�TCaCO3��+2HClO��
��5��������ƺ��Ȼ��Ƶ����ʵ���Ũ�Ⱦ�Ϊ0.01mol/L��������Һ�������Ϊ1L������Һ�к��д�����ƺ��Ȼ��Ƶ����ʵ�����Ϊ0.01mol������m=n��M�������������Ϊm=n��M=0.01mol��143g/mol=1.43g���Ȼ��Ƶ�����Ϊm=n��M=0.01mol��111g/mol=1.11g��Ư�۲����������ʣ��ɷ�Ϊ������ƺ��Ȼ��ƣ�����Ư�۵�����Ϊ2.54g��
�ʴ�Ϊ��2.54g��
���������⿼��Ư�۵����ʡ��Ʊ���Ӧ�ã�ΪԪ�ػ������г��������⣬�����ڻ���֪ʶ�Ŀ��飬ע��������ʵ��Ʊ��������Լ�Ӧ�ã�ѧϰ��ע����ػ���֪ʶ�Ļ��ۣ���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
 ����A��B��C���ֶ�����Ԫ�أ�ԭ���������ε�����A��C��������֮��Ϊ27������������֮��Ϊ5��0.9g����B���������ᷴӦ���ռ�������1.12L����״��������ش��������⣺
����A��B��C���ֶ�����Ԫ�أ�ԭ���������ε�����A��C��������֮��Ϊ27������������֮��Ϊ5��0.9g����B���������ᷴӦ���ռ�������1.12L����״��������ش��������⣺

