��Ŀ����
����F��ˮ��Һ������A��D���ʻ�ɫ��D�ǵ��ʣ�F����Һ����������ɫ��A��һ����Ҫ��ҵ�����е���Ҫԭ�ϡ���A��C��D������ijһ�ǽ���Ԫ�أ�C����Է�������������������Է���������ȡ���Ӧ���������ɵ�ˮ����ת���ص������Ѿ���ȥ����Щ����������ת����ϵ���Իش��������⣺
(1)A��_________��C��_________��F��_________(���ѧʽ)��
(2)A�������ᷴӦ�����ӷ���ʽΪ_________��
(3)д���ÿ�ͼ����B��E�Ļ�ѧ����ʽ_________��
(1)FeS2 H2S FeCl3
(2)FeS2+2H+![]() Fe2++H2S��+S��
Fe2++H2S��+S��
(3)FeCl2+2NaOH![]() Fe(OH)2��+2NaCl
Fe(OH)2��+2NaCl
4Fe(OH)2+O2+2H2O![]() 4Fe(OH)3
4Fe(OH)3
�����������⿼��������ƶϣ��е��⡣ץסͻ�ƿڡ�D�ǵ����ҳʻ�ɫ���������ƶ�DΪS�����ݡ�A��C��D������ijһ�ǽ���Ԫ�ء���������SԪ�أ���C����Է�������������������Է���������ȣ���CΪH2S��F����Һ����������ɫ����FΪFeCl3����AΪFeS2��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ


 2BO3����һ���̶��ݻ�Ϊ2L���ܱ������г���0.20mol��BO2��0.10mol��O2������Ӻ�ﵽƽ�⣬��������к�BO3Ϊ0.18mol����v��o2 ��=
2BO3����һ���̶��ݻ�Ϊ2L���ܱ������г���0.20mol��BO2��0.10mol��O2������Ӻ�ﵽƽ�⣬��������к�BO3Ϊ0.18mol����v��o2 ��=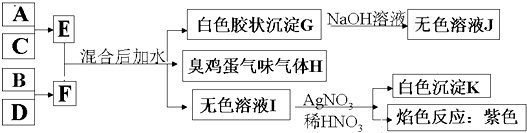
 2BO3����һ���̶��ݻ�Ϊ2L���ܱ������г���0.20mol��BO2��0.10mol��O2������Ӻ�ﵽƽ�⣬��������к�BO3Ϊ0.18mol����v��o2 ��=______mol?L-1?min-1��������ͨ��0.20mol BO2��0.10mol O2���ٴδﵽ��ƽ���BO3�����ʵ�������______֮�䣮
2BO3����һ���̶��ݻ�Ϊ2L���ܱ������г���0.20mol��BO2��0.10mol��O2������Ӻ�ﵽƽ�⣬��������к�BO3Ϊ0.18mol����v��o2 ��=______mol?L-1?min-1��������ͨ��0.20mol BO2��0.10mol O2���ٴδﵽ��ƽ���BO3�����ʵ�������______֮�䣮
 2BO3����һ���̶��ݻ�Ϊ2L���ܱ������г���0.20 mol��BO2��0.10mol��O2������Ӻ�ﵽƽ�⣬��������к�BO3Ϊ0.18mol����
2BO3����һ���̶��ݻ�Ϊ2L���ܱ������г���0.20 mol��BO2��0.10mol��O2������Ӻ�ﵽƽ�⣬��������к�BO3Ϊ0.18mol���� = ________ mol��L-1��min-1��������ͨ��0.20mol BO2��0.10mol O2���ٴδﵽ��ƽ���BO3�����ʵ�������__________֮�䡣
= ________ mol��L-1��min-1��������ͨ��0.20mol BO2��0.10mol O2���ٴδﵽ��ƽ���BO3�����ʵ�������__________֮�䡣 