��Ŀ����
A��B��C��D����Ԫ��ԭ�ӵĺ˵������������С��20�����䵥�ʼ���Ӧ�Ļ������ܷ������·�Ӧ��ϵ��

��1��д��F�ĵ���ʽ______��
��2������H�ж�������Na2CO3��Һ���գ�����������ʽ�Σ��÷�Ӧ�Ļ�ѧ����ʽΪ��______��
��3������E��ˮ��Һ�����գ����յõ��Ĺ���Ϊ______��ԭ��Ϊ______��______�����û�ѧ��Ӧ����ʽ����ʾ��
��4�������£���F��ˮ��Һ�м���������Ũ�ȵ����ᷴӦ��������Һ��PH��7�������Һ������Ũ����С�����˳��Ϊ��______��
��5��������H���ڿ����г��ȼ�տɵõ�����������BO2��BO2��������������Ӧ��2BO2+O2  2BO3����һ���̶��ݻ�Ϊ2L���ܱ������г���0.20mol��BO2��0.10mol��O2������Ӻ�ﵽƽ�⣬��������к�BO3Ϊ0.18mol����v��o2 ��=______mol?L-1?min-1��������ͨ��0.20mol BO2��0.10mol O2���ٴδﵽ��ƽ���BO3�����ʵ�������______֮�䣮
2BO3����һ���̶��ݻ�Ϊ2L���ܱ������г���0.20mol��BO2��0.10mol��O2������Ӻ�ﵽƽ�⣬��������к�BO3Ϊ0.18mol����v��o2 ��=______mol?L-1?min-1��������ͨ��0.20mol BO2��0.10mol O2���ٴδﵽ��ƽ���BO3�����ʵ�������______֮�䣮
�⣺A��B��C��D����Ԫ��ԭ�ӵĺ˵������С��20��G�ǰ�ɫ��״��������������������Һ��GΪAl��OH��3��JΪNaAlO2��HΪ��������ζ�����壬ΪH2S����ɫ��ҺI�������������ᷴӦ���ɰ�ɫ����K��KΪAgCl��������ɫ��ӦΪ��ɫ����IΪKCl��
����Ԫ���غ㣬���E+F Al��OH��3+H2S+KCl����֪A��B��C��D����Ԫ��ΪAl��S��Cl��K��A��B��C��D����Ԫ��ԭ�ӵĺ˵������������AΪAl��BΪS��CΪCl��DΪK����A+C��E��֪��EΪAlCl3����B+D��F��֪��FΪK2S��
Al��OH��3+H2S+KCl����֪A��B��C��D����Ԫ��ΪAl��S��Cl��K��A��B��C��D����Ԫ��ԭ�ӵĺ˵������������AΪAl��BΪS��CΪCl��DΪK����A+C��E��֪��EΪAlCl3����B+D��F��֪��FΪK2S��
��1��FΪK2S���������ӻ�����ɼ������������ӹ��ɣ�����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��2��H2S�ж�������Na2CO3��Һ���գ�����������ʽ�Σ�ΪNaHS��NaHCO3����Ӧ����ʽΪ��H2S+Na2CO3=NaHS+NaHCO3��
�ʴ�Ϊ��H2S+Na2CO3=NaHS+NaHCO3��
��3��EΪAlCl3��AlCl3��ˮ��Һ�д���ˮ��ƽ��AlCl3+3H2O?Al��OH��3+3HCl������AlCl3��ˮ��Һ��HCl�ӷ����ٽ�ˮ��̶Ƚ��У��õ�Al��OH��3������Al��OH��3��Al��OH��3�ֽ�2Al��OH��3 Al2O3+2H2O�����յõ��Ĺ���ΪAl2O3��
Al2O3+2H2O�����յõ��Ĺ���ΪAl2O3��
�ʴ�Ϊ��Al2O3��AlCl3+3H2O?Al��OH��3+3HCl��2Al��OH��3 Al2O3+2H2O��
Al2O3+2H2O��
��4�������£���K2S��ˮ��Һ�м���������Ũ�ȵ����ᣬK2S��HCl���ʵ�����ȣ�������ӦK2S+HCl=KHS+KCl����Ϻ���ҺΪ��Ũ�ȵ�KHS��KCl��Һ����Һ��PH��7����[H+]��[OH-]��˵��HS-��ˮ��̶ȴ��ڵ���̶ȣ���[HS-]��[Cl-]��ˮ��̶Ⱥ�С����[OH-]��[HS-]����Һ��H+����ˮ�ĵ�����HS-���룬����[S2-]��[H+]����Һ��K+Ũ�������Һ������Ũ����С�����˳��Ϊ��[S2-]��[H+]��[OH-]��[HS-]��[Cl-]��[K+]��
�ʴ�Ϊ��[S2-]��[H+]��[OH-]��[HS-]��[Cl-]��[K+]��
��5����һ���̶��ݻ�Ϊ2L���ܱ������г���0.20mol��SO2��0.10mol��O2������Ӻ�ﵽƽ�⣬��������к�SO3Ϊ0.18mol��v��SO3��= =0.18mol/��L?min��������֮�ȵ��ڻ�ѧ������֮�ȣ���v��O2 ��=
=0.18mol/��L?min��������֮�ȵ��ڻ�ѧ������֮�ȣ���v��O2 ��=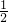 v��SO3��=
v��SO3��=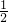 ��0.18mol/��L?min��=0.09mol/��L?min����
��0.18mol/��L?min��=0.09mol/��L?min����
������ͨ��0.20mol SO2��0.10mol O2��ѹǿ��ǿ��ƽ��������Ӧ�ƶ�����Ӧת���������ٴδﵽ��ƽ���SO3�����ʵ�������0.18mol��2=0.36mol����0.4molSO2��0.2molO2��ȫ��Ӧ����������0.4molSO3����Ӧ�ﲻ����ȫת�����ʴ�SO3С��0.4mol����SO3�����ʵ�������0.36mol��0.40mol֮�䣻
�ʴ�Ϊ��0.09��0.36mol��0.40mol��
������A��B��C��D����Ԫ��ԭ�ӵĺ˵������С��20��G�ǰ�ɫ��״��������������������Һ��GΪAl��OH��3��JΪNaAlO2��HΪ��������ζ�����壬ΪH2S����ɫ��ҺI�������������ᷴӦ���ɰ�ɫ����K��KΪAgCl��������ɫ��ӦΪ��ɫ����IΪKCl��
����Ԫ���غ㣬���E+F Al��OH��3+H2S+KCl����֪A��B��C��D����Ԫ��ΪAl��S��Cl��K��A��B��C��D����Ԫ��ԭ�ӵĺ˵������������AΪAl��BΪS��CΪCl��DΪK����A+C��E��֪��EΪAlCl3����B+D��F��֪��FΪK2S��
Al��OH��3+H2S+KCl����֪A��B��C��D����Ԫ��ΪAl��S��Cl��K��A��B��C��D����Ԫ��ԭ�ӵĺ˵������������AΪAl��BΪS��CΪCl��DΪK����A+C��E��֪��EΪAlCl3����B+D��F��֪��FΪK2S��
����������������ͼ�����ʽ����Al��S��Cl��Ԫ�ص��ʼ��仯����֮����ת����ϵ����������������ѧ�������д������ˮ�⡢��ѧ��Ӧ�����뻯ѧƽ��ļ���ȣ�������HΪ��������ζ�����壬G�ǰ�ɫ��״������I��Һ��ɫ��ӦΪ��ɫ�Ⱦ�Ϊ����ͻ�ƿڣ���4��������Ũ�ȵıȽ����ѵ����״��㣬��Ŀ�ۺ��Խϴ��ѶȽϴ�
����Ԫ���غ㣬���E+F
 Al��OH��3+H2S+KCl����֪A��B��C��D����Ԫ��ΪAl��S��Cl��K��A��B��C��D����Ԫ��ԭ�ӵĺ˵������������AΪAl��BΪS��CΪCl��DΪK����A+C��E��֪��EΪAlCl3����B+D��F��֪��FΪK2S��
Al��OH��3+H2S+KCl����֪A��B��C��D����Ԫ��ΪAl��S��Cl��K��A��B��C��D����Ԫ��ԭ�ӵĺ˵������������AΪAl��BΪS��CΪCl��DΪK����A+C��E��֪��EΪAlCl3����B+D��F��֪��FΪK2S����1��FΪK2S���������ӻ�����ɼ������������ӹ��ɣ�����ʽΪ��
 ��
���ʴ�Ϊ��
 ��
����2��H2S�ж�������Na2CO3��Һ���գ�����������ʽ�Σ�ΪNaHS��NaHCO3����Ӧ����ʽΪ��H2S+Na2CO3=NaHS+NaHCO3��
�ʴ�Ϊ��H2S+Na2CO3=NaHS+NaHCO3��
��3��EΪAlCl3��AlCl3��ˮ��Һ�д���ˮ��ƽ��AlCl3+3H2O?Al��OH��3+3HCl������AlCl3��ˮ��Һ��HCl�ӷ����ٽ�ˮ��̶Ƚ��У��õ�Al��OH��3������Al��OH��3��Al��OH��3�ֽ�2Al��OH��3
 Al2O3+2H2O�����յõ��Ĺ���ΪAl2O3��
Al2O3+2H2O�����յõ��Ĺ���ΪAl2O3�� �ʴ�Ϊ��Al2O3��AlCl3+3H2O?Al��OH��3+3HCl��2Al��OH��3
 Al2O3+2H2O��
Al2O3+2H2O����4�������£���K2S��ˮ��Һ�м���������Ũ�ȵ����ᣬK2S��HCl���ʵ�����ȣ�������ӦK2S+HCl=KHS+KCl����Ϻ���ҺΪ��Ũ�ȵ�KHS��KCl��Һ����Һ��PH��7����[H+]��[OH-]��˵��HS-��ˮ��̶ȴ��ڵ���̶ȣ���[HS-]��[Cl-]��ˮ��̶Ⱥ�С����[OH-]��[HS-]����Һ��H+����ˮ�ĵ�����HS-���룬����[S2-]��[H+]����Һ��K+Ũ�������Һ������Ũ����С�����˳��Ϊ��[S2-]��[H+]��[OH-]��[HS-]��[Cl-]��[K+]��
�ʴ�Ϊ��[S2-]��[H+]��[OH-]��[HS-]��[Cl-]��[K+]��
��5����һ���̶��ݻ�Ϊ2L���ܱ������г���0.20mol��SO2��0.10mol��O2������Ӻ�ﵽƽ�⣬��������к�SO3Ϊ0.18mol��v��SO3��=
 =0.18mol/��L?min��������֮�ȵ��ڻ�ѧ������֮�ȣ���v��O2 ��=
=0.18mol/��L?min��������֮�ȵ��ڻ�ѧ������֮�ȣ���v��O2 ��=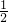 v��SO3��=
v��SO3��=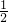 ��0.18mol/��L?min��=0.09mol/��L?min����
��0.18mol/��L?min��=0.09mol/��L?min����������ͨ��0.20mol SO2��0.10mol O2��ѹǿ��ǿ��ƽ��������Ӧ�ƶ�����Ӧת���������ٴδﵽ��ƽ���SO3�����ʵ�������0.18mol��2=0.36mol����0.4molSO2��0.2molO2��ȫ��Ӧ����������0.4molSO3����Ӧ�ﲻ����ȫת�����ʴ�SO3С��0.4mol����SO3�����ʵ�������0.36mol��0.40mol֮�䣻
�ʴ�Ϊ��0.09��0.36mol��0.40mol��
������A��B��C��D����Ԫ��ԭ�ӵĺ˵������С��20��G�ǰ�ɫ��״��������������������Һ��GΪAl��OH��3��JΪNaAlO2��HΪ��������ζ�����壬ΪH2S����ɫ��ҺI�������������ᷴӦ���ɰ�ɫ����K��KΪAgCl��������ɫ��ӦΪ��ɫ����IΪKCl��
����Ԫ���غ㣬���E+F
 Al��OH��3+H2S+KCl����֪A��B��C��D����Ԫ��ΪAl��S��Cl��K��A��B��C��D����Ԫ��ԭ�ӵĺ˵������������AΪAl��BΪS��CΪCl��DΪK����A+C��E��֪��EΪAlCl3����B+D��F��֪��FΪK2S��
Al��OH��3+H2S+KCl����֪A��B��C��D����Ԫ��ΪAl��S��Cl��K��A��B��C��D����Ԫ��ԭ�ӵĺ˵������������AΪAl��BΪS��CΪCl��DΪK����A+C��E��֪��EΪAlCl3����B+D��F��֪��FΪK2S������������������ͼ�����ʽ����Al��S��Cl��Ԫ�ص��ʼ��仯����֮����ת����ϵ����������������ѧ�������д������ˮ�⡢��ѧ��Ӧ�����뻯ѧƽ��ļ���ȣ�������HΪ��������ζ�����壬G�ǰ�ɫ��״������I��Һ��ɫ��ӦΪ��ɫ�Ⱦ�Ϊ����ͻ�ƿڣ���4��������Ũ�ȵıȽ����ѵ����״��㣬��Ŀ�ۺ��Խϴ��ѶȽϴ�

��ϰ��ϵ�д�
 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д�
�����Ŀ




 ��B��C���γ����ӻ�����
��B��C���γ����ӻ����� ��B��C���γ����ӻ�����
��B��C���γ����ӻ�����