��Ŀ����
10�������д����л�ѧ����1������ѡ����ʳ����ȷʹ��ҩ���DZ�֤���Ľ�������Ҫ���棬����д���пո�
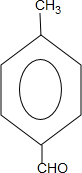
ˮ������������ʮ����Ҫ�����ã�������Ϊ�ܼ��������������£�����һ�ֱز����ٵķ�Ӧ���д����������������ȫˮ��ķ�Ӧ����ʽnH2O+��C6H10O5��n��nC6H12O6����ÿ�춼Ҫ����һ������ˮ��������ҺҲ���Լ������ʣ���������������ѪҺ�������ԣ�ѡ������ԣ����ԣ������ԣ����ҹ������γ��������߲˺�ˮ��ƫ�ٵ�ϰ�ߣ�һ����Һ�������ԣ�ѡ������ԣ����ԣ������ԣ���ͨ��ʳ��ĵ�����������Ա��ֽ�����������������ˣ����ǻ���ҪȥҽԺ���ҽ��������ҩһ����R��ǣ���ʾ����ҩ����ù�ص�����Ƚ϶࣬ʹ�õ��ǰ�����ù��ǰһ��Ҫ����Ƥ�ԣ�Ƥ���������飩����˾ƥ�ֵĽṹ��ʽΪ
 ���еĹ��������������Ȼ���д���ƣ����ð�˾ƥ�ֺ����������к��Ƶÿ�����˾ƥ�֣��÷�Ӧ�Ļ�ѧ����ʽΪC6H5OOCCH3COOH+H2O��CH3COOH+C6H5OHCOOH��
���еĹ��������������Ȼ���д���ƣ����ð�˾ƥ�ֺ����������к��Ƶÿ�����˾ƥ�֣��÷�Ӧ�Ļ�ѧ����ʽΪC6H5OOCCH3COOH+H2O��CH3COOH+C6H5OHCOOH����2�����������������������Ҫ���ʻ���������ѧ�Dz��Ͽ�ѧ��չ�Ļ���������д���пո�
A�������еIJ������մɡ�ˮ�����ڴ�ͳ�������β��ϣ��������������ࣩ
B���Ͻ���ϸ������Fe��C�ڳ�ʪ�Ŀ�����������ԭ��ض������绯ѧ��ʴ��������Ӧ����ʽΪO2+2H2O+4e-=4OH-��
C������װ�����绯�˵�̺�����ϰ�Ⱦ����ͷų���Ⱦ�����ļ�ȩ���壮
D������ϩ���ճ������п��Ƴ�ʳƷ����Ĥ��
F���ִ���ʯ�ͻ���Ϊ����������ϳɲ��������ϡ��ϳ���ά���ϳ���
��3�����������Ϊ�˱��������������ھ�������������˵����ȷ����BCD
A��CO���ױ�����Ŷ��Ȳ���������Ⱦ��
B����ˮ����������ѧ�������������кͷ���������
C������ɫ��Ⱦ���Ļ�Σ���������������
D��������Դ���ķ����Ƿ�����շ�
E��Ϊ��ֹ���д�Ⱦ���ɽ������Ŵ��رպ��ü�ȩѬ������������
F��Ǧ��о��ԭ�ϵ��ؽ���Ǧ����ͯ��ʹ��ʱ��������˱ҧǦ�ʣ���������Ǧ�ж�
G��CO�ж�������ú¯�ľ��ң��ɷ�������ˮ��������Ч������CO���Է�ú���ж���
���� ��1������ˮ�����������ǣ��˵�ѪҺ�������ԣ��߲˺�ˮ���Ǽ���ʳ���ù���Ǵ���ҩ��ijЩ��Ⱥ����ù�ع����� �����������Ȼ�����˾ƥ��ˮ��������������ǻ������
�����������Ȼ�����˾ƥ��ˮ��������������ǻ������
��2��A������ͳ�����β�ƷΪ���������մɡ�ˮ�ࣻ
B���Ͻ���ϸ������Fe��C�ڳ�ʪ�Ŀ����з���������ʴ��
C������װ�������ͷ�����ӷ��Ե��л�����ȩ�ȣ�
D���ճ�����������ʳƷ����Ĥ�õ��Ǿ���ϩPE��
F������ϳɲ���Ϊ�����ϡ��ϳ���ά���ϳ���
��3��A��CO���ױ��Լ���������������Ŷ������嶼�к�������������Ⱦ�
B�����ݳ��õĻ�ѧ�������У��к͡�������������������ԭ�ȷ�����������
C����ɫ��Ⱦ���ڶѻ����ƻ���������Ⱦ����ˮ��Σ��������������棬��ɺ����¼��ȣ�
D�����������Ƿ��Ϊ������Դ�����ٻ�����Ⱦ��
E����ȩ�������к���
F��Ǧ��о����Ҫ�ɷ���ճ����ʯī��
G��һ����̼������ˮ��
��� �⣺��1������ˮ�����������ǣ�nH2O+��C6H10O5��n��nC6H12O6���߲˺�ˮ���Ǽ���ʳ��������ڴ�л�ʼ��ԣ��ǰ�����ù���Ǵ���ҩ��ijЩ��Ⱥ����ù�ع�����ʹ����ù��ǰһ��Ҫ����Ƥ���������飻 �����������Ȼ�����˾ƥ��ˮ��������������ǻ������C6H5OOCCH3COOH+H2O��CH3COOH+C6H5OHCOOH��
�����������Ȼ�����˾ƥ��ˮ��������������ǻ������C6H5OOCCH3COOH+H2O��CH3COOH+C6H5OHCOOH��
�ʴ�Ϊ��nH2O+��C6H10O5��n��nC6H12O6�������ԣ������ԣ�����ҩ��Ƥ�ԣ�Ƥ���������飩���Ȼ���C6H5OOCCH3COOH+H2O��CH3COOH+C6H5OHCOOH��
��2��A������ͳ�����β�ƷΪ���������մɡ�ˮ�࣬�ʴ�Ϊ���������մɣ�ˮ�ࣻ
B���Ͻ���ϸ������Fe��C�ڳ�ʪ�Ŀ����з���������ʴ�������缫��Ӧʽ��O2+2H2O+4e-=4OH-���ʴ�Ϊ��O2+2H2O+4e-=4OH-��
C������װ�������ͷ�����ӷ��Ե��л�����ȩ���ʴ�Ϊ����ȩ��
D���ճ�����������ʳƷ����Ĥ�õ��Ǿ���ϩPE���ʴ�Ϊ������ϩ��
F�����ϡ��ϳ���ά���ϳ���Ϊ����ϳɲ��ϣ��ʴ�Ϊ�����ϣ��ϳ���ά���ϳ���
��3��A�����̲�����������һ����̼����Ŷ���װ�β������ϼ��ͷŵ����ʼױ������ʣ������嶼�к�������������Ⱦ���A����
B����ˮ�����������������������кͷ�����������������ԭ������B��ȷ��
C����ɫ��Ⱦ���ڶѻ���Σ��������������棬��ɺ����¼�����C��ȷ��
D��������������գ�����������Ŀ�ľ���Ҫ����Σ����������Դ�����ú�������������D��ȷ��
E����ȩ�������к��������ü�ȩ��ȩѬ������������������ʳ�ף����к�ǿ��ɱ������������ϸ����������ֹ���д�Ⱦ����E����
F��Ǧ�ʵı�о����ʯī��ճ����һ����������Ƴɵģ����ǽ���Ǧ����F����
G��һ����̼������ˮ��ˮ��������CO�����Բ��ܷ�ú���ж�����G����
��ѡ��BCD��
���� ���⿼�������³´�л�����е�������Ӧ����ѧ����ʽ����д����������β�Ʒ������ϳɲ��ϣ����漰������������ʴ��ʳƷ����Ĥ����ԭ�ϣ�װ���ϵ�Σ����֪ʶ���缫��Ӧʽ����дʱ�ѵ㣬��������������ϵ���У���Ŀ�ѶȲ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ���ʣ��ޢ� | B�� | ������ڢ� | C�� | ��٢� | D�� | �л���ۢ� |
| A�� | 1molCl2��Ϊ�������õ��ĵ�����ΪNA | |
| B�� | ��0�棬101kPaʱ��22.4L�����к���NA����ԭ�� | |
| C�� | 25�棬1.01��105Pa��64gSO2�к��е�ԭ����Ϊ3NA | |
| D�� | NA��һ����̼���Ӻ�0.5mol�����������Ϊ7��4 |
| A�� | 0.1molZn�뺬0.1molHCl�������ַ�Ӧ��ת�Ƶĵ�����ĿΪ0.2NA | |
| B�� | 1molNa������O2��Ӧ������Na2O��Na2O2�Ļ���ת�Ƶĵ�����ΪNA | |
| C�� | 1molNa2O2������CO2��ַ�Ӧת�Ƶĵ�����Ϊ2NA | |
| D�� | ��FeI2��Һ��ͨ������Cl2������1molFe2+������ʱ����ת�Ƶĵ��ӵ���ĿΪNA |
| A�� | ��״���£�22.4LH2O���еķ�����Ϊ1 NA | |
| B�� | ���³�ѹ�£�22g CO2����ԭ����1.5NA | |
| C�� | 32g �����ͳ����Ļ�������к�����ԭ�ӵĸ���Ϊ2NA | |
| D�� | ���ʵ���Ũ��Ϊ0.5mol/L��MgCl2��Һ�У�����Cl- ����Ϊ1 NA |
| A�� | CH3CH3+Cl2 $\stackrel{����}{��}$CH3CH2Cl+HCl | |
| B�� | CH2�TCH2+HBr��CH3CH2Br | |
| C�� | 2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O | |
| D�� | CH3COOH+CH3CH2OH$?_{��}^{Ũ����}$H3COOCH2CH3+H2O |



 ��
�� +2Cu��OH��2$\stackrel{��}{��}$
+2Cu��OH��2$\stackrel{��}{��}$ +Cu2O��+2H2O��
+Cu2O��+2H2O�� $\stackrel{һ��������}{��}$
$\stackrel{һ��������}{��}$ +nH2O��
+nH2O��
 ��
�� A��B��C��D��E��ԭ���������εݼ������ֳ���Ԫ�أ�CԪ���ǵؿ��к�������Ԫ�أ�D��E��ɵ���̬������M��ˮ��Һ�ʼ��ԣ�B�ĵ�����C2��ȼ�ղ����ʹƷ����Һ��ɫ��A��һ����ʷ�ƾá�Ӧ�ù㷺�Ľ���Ԫ�أ���ش�
A��B��C��D��E��ԭ���������εݼ������ֳ���Ԫ�أ�CԪ���ǵؿ��к�������Ԫ�أ�D��E��ɵ���̬������M��ˮ��Һ�ʼ��ԣ�B�ĵ�����C2��ȼ�ղ����ʹƷ����Һ��ɫ��A��һ����ʷ�ƾá�Ӧ�ù㷺�Ľ���Ԫ�أ���ش� ��
��