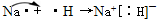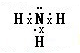��Ŀ����
ͼ��Ԫ�����ڱ��Ŀ�ܣ�����Ԫ�����ڱ��ش��������⣺
��1�����ڱ��е�Ԫ�آݺ�Ԫ�آ�����������ˮ�������ǿ��˳����
��2����д���ڵ��⻯��ĵ���ʽ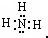
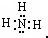 ���۵��⻯��Ľṹʽ
���۵��⻯��Ľṹʽ
��3���١���Ԫ�صĵ��ʣ��ڳ����»�ѧ�����ȶ���ͨ������������������
��4����д����ҵ���Ʊ��ߵ��ʵĻ�ѧ����ʽ

��1�����ڱ��е�Ԫ�آݺ�Ԫ�آ�����������ˮ�������ǿ��˳����
NaOH��Mg��OH��2
NaOH��Mg��OH��2
���û�ѧʽ��ʾ�������ڱ��е�Ԫ�آܺ�Ԫ�آߵ��⻯��ķе�ߵ�˳����HF��HCl
HF��HCl
���û�ѧʽ��ʾ������2����д���ڵ��⻯��ĵ���ʽ
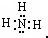
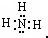
H-O-H
H-O-H
����3���١���Ԫ�صĵ��ʣ��ڳ����»�ѧ�����ȶ���ͨ������������������
N2
N2
����д����ʽ������4����д����ҵ���Ʊ��ߵ��ʵĻ�ѧ����ʽ
2NaCl+2H2O
2NaOH+H2��+Cl2��
| ||
2NaCl+2H2O
2NaOH+H2��+Cl2��
��
| ||
��������1������Ԫ�����ڱ��Ľṹ��ͬ���ڡ�ͬ����Ԫ�����ʵĵݱ����˼����
��2�����ݻ�ѧ�����۷�����
��3���������ʵ����ʽ��
��4�����ݵ�ⱥ��ʳ��ˮ��ԭ��ȥ��д��
��2�����ݻ�ѧ�����۷�����
��3���������ʵ����ʽ��
��4�����ݵ�ⱥ��ʳ��ˮ��ԭ��ȥ��д��
����⣺��1������Ԫ�����ڱ����ݡ��ֱ��ڵ������ڵ�IA��IIA����ݡ��ֱ�ΪNa��Mg��ͬ���ڴ�����Ԫ����߾��к�ǿ��Ӧˮ����ļ������μ���������ǿ��˳��ΪNaOH��Mg��OH��2��
�ܡ��߷ֱ���VIIA�ĵڶ����ں͵������ڣ���ܡ��߷ֱ�ΪF��Cl������HF�в������ڷ��Ӽ����������������������HCl��ֻ���ڷ��Ӽ�������������е�ߵ�˳����HF��HCl��
��2������Ԫ�����ڱ�������N����O�����ݹ��ۼ������ۣ�NH3�ĵ���ʽΪ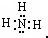 ��H2O�ĽṹʽΪ��H-O-H��
��H2O�ĽṹʽΪ��H-O-H��
��3���١��߷ֱ�ΪH��N��O��F��Na��Mg��Cl���䵥������������õ���N2������������������
��4����ҵ����ȡ�����õ��ǵ�ⱥ��ʳ��ˮ�ķ������仯ѧ����ʽΪ��2NaCl+2H2O
2NaOH+H2��+Cl2����
�ʴ�Ϊ��
��1��NaOH��Mg��OH��2��HF��HCl
��2��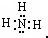 ��H-O-H
��H-O-H
��3��N2��
��4��2NaCl+2H2O
2NaOH+H2��+Cl2����
�ܡ��߷ֱ���VIIA�ĵڶ����ں͵������ڣ���ܡ��߷ֱ�ΪF��Cl������HF�в������ڷ��Ӽ����������������������HCl��ֻ���ڷ��Ӽ�������������е�ߵ�˳����HF��HCl��
��2������Ԫ�����ڱ�������N����O�����ݹ��ۼ������ۣ�NH3�ĵ���ʽΪ
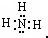 ��H2O�ĽṹʽΪ��H-O-H��
��H2O�ĽṹʽΪ��H-O-H����3���١��߷ֱ�ΪH��N��O��F��Na��Mg��Cl���䵥������������õ���N2������������������
��4����ҵ����ȡ�����õ��ǵ�ⱥ��ʳ��ˮ�ķ������仯ѧ����ʽΪ��2NaCl+2H2O
| ||
�ʴ�Ϊ��
��1��NaOH��Mg��OH��2��HF��HCl
��2��
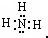 ��H-O-H
��H-O-H��3��N2��
��4��2NaCl+2H2O
| ||
���������⽫Ԫ�����ڱ���֪ʶ��Ԫ�����ʵĵݱ���ɽ����һ�𣬾��к�ǿ���ۺ��ԣ��ѶȽϴ���Ҫ����������ۺ�˼�����ܺܺõ�ѵ��˼ά������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ