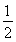��Ŀ����
������أ� ������ǿ�����ԣ�������ԭΪ����أ���80 �����������ֽ⡣ʵ����ģ�ҵ�ϳɹ�����ص��������£�
������ǿ�����ԣ�������ԭΪ����أ���80 �����������ֽ⡣ʵ����ģ�ҵ�ϳɹ�����ص��������£�

��1������狀��������Ƴɵ��Һ���Բ����缫���е�⣬���ɹ��������Һ��д�����ʱ������Ӧ�����ӷ���ʽ_____________________________________
___________________________________��
��2����֪������ʵ��ܽ����������ͼ��ʾ����ʵ�������ᴿ������شֲ�Ʒ��ʵ������������Ϊ����������شֲ�Ʒ��������ˮ�У�________________�����

��3����Ʒ�й�����صĺ������õ��������вⶨ��ʵ�鲽�����£�
����1����ȡ���������Ʒ0.300 0 g�ڵ���ƿ�У�����30 mLˮ�ܽ⡣
����2������Һ�м���4.00 0 g KI���壨�Թ�������ҡ�ȣ��ڰ�������30 min��
����3���ڵ���ƿ�м�������������Һ�ữ���Ե�����Һ��ָʾ������0.100 0 mol��L��1Na2S2O3����Һ�ζ����յ㣬������Na2S2O3����Һ21.00 mL��
����֪��Ӧ��I2��2S2O32-=2I����S4O62-��
��������2��δ������ƿ���ڰ�������30 min�����������в���3����ⶨ�Ľ������________��ѡ����ƫ��������ƫС��������Ӱ����������������3�еζ��յ��������____________________________________________��
��������������ɼ��������Ʒ�й�����ص���������Ϊ_______________��
��Ϊȷ��ʵ������ȷ�ԣ�����Ϊ����Ҫ____________________________��
��4����0.40 mol���������0.20 mol�������Ƴ�1 L��Һ����80 �������¼��Ȳ���tʱ������Һ�еμ�������FeCl3��Һ���ⶨ��Һ�и��ɷֵ�Ũ����ͼ��ʾ��H��Ũ��δ��������ͼ������X�Ļ�ѧʽΪ________________________��

��1��2SO42-��2H�� S2O82-��H2��
S2O82-��H2��
��2���ڲ�����80 ���������¼���Ũ������ȴ�ᾧ�����ˣ�����ˮϴ�ӡ���3����ƫС�����һ�ε������Һ����ɫ��Ϊ��ɫ����30 s����ɫ����94.50%����0.945 0��
���ظ�����ʵ�鲽��1��2�Ρ���4��H2O2
����������1��������֪�������ת��Ϊ�������Ϊ������Ӧ����Ӧ����H���Ļ�ԭ��Ӧ����2�����ݸ����ʵ��ܽ�ȱ仯������ص����ʣ�Ӧ�����ؽᾧ�������ᴿ���롣��3�������ڲ���2��δ������ƿ����30 min�������в���3���������ɹ������δ����ȫת�������յ��²ⶨ���ƫС���ζ��յ㼴�ǵⵥ�ʸպ�������ϣ����һ�ε������Һ����ɫ��Ϊ��ɫ����30 s����ɫ�������ݵ���ת�ƹ�ϵ��S2O82-��I2��2S2O32-����n��K2S2O8����0.1000 mol��L��1��0.021 L�� ��0.001 05 mol��m��K2S2O8����0.001 05 mol��270 g��mol��1��0.283 5 g��w��K2S2O8����
��0.001 05 mol��m��K2S2O8����0.001 05 mol��270 g��mol��1��0.283 5 g��w��K2S2O8���� ��100%��94.50%��������ʵ���Ӧ�ظ�����2��3�Σ���Ӧ�����ظ�����ʵ�鲽��1��2��������4���ɹ�����صĽṹ��ͼ�����ӵ����仯֪��0.4 mol������ؿ�����0.4 mol H2O2��Ȼ���ٷֽ�ɵ�0.2 mol O2����ͼ�Ǻϡ�
��100%��94.50%��������ʵ���Ӧ�ظ�����2��3�Σ���Ӧ�����ظ�����ʵ�鲽��1��2��������4���ɹ�����صĽṹ��ͼ�����ӵ����仯֪��0.4 mol������ؿ�����0.4 mol H2O2��Ȼ���ٷֽ�ɵ�0.2 mol O2����ͼ�Ǻϡ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�T Kʱ����2.0 L�����ܱ������г���1.0 mol COCl2����ӦCOCl2��g�� Cl2��g����CO��g��������һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±���
Cl2��g����CO��g��������һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±���
t/s | 0 | 2 | 4 | 6 | 8 |
n��Cl2��/mol | 0 | 0.16 | 0.19 | 0.20 | 0.20 |
����˵����ȷ���ǣ���������
A����Ӧ��ǰ2 s ��ƽ������v��CO����0.080 mol��L��1��s��1
B�����������������䣬�����¶ȣ�ƽ��ʱ��c��Cl2����0.11 mol��L��1����Ӧ����H��0
C��T Kʱ��ʼ�������г���0.9 mol COCl2��0.10 mol Cl2 ��0.10 mol CO����Ӧ�ﵽƽ��ǰv����v��
D��T Kʱ��ʼ�������г���1.0 mol Cl2��0.9 mol CO���ﵽƽ��ʱ��Cl2��ת����С��80%