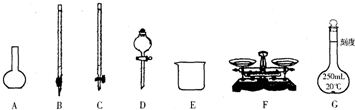
��Ŀ����
50mL 1.0mol?L-1�����50mL 1.1mol?L-1����������Һ��ͼ1װ���н����кͷ�Ӧ����ͨ���ⶨ��Ӧ���������ų��������������к��ȣ��Իش��������⣺
��1����С�ձ�����������ĭ���ϵ�����
��2��
��3�����ձ����粻��Ӳֽ�壬������к�����ֵ��Ӱ����
��4���������60mL 1.0mol?L-1�����50mL 1.1mol?L-1����������Һ���з�Ӧ����������ʵ����ȣ���������
��5��ij�о�С�齫װ�����ƺ�V1 mL 1.0mol/L HCl��Һ��V2 mLδ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ��������¼��Һ�¶ȣ�ʵ������ͼ2��ʾ��ʵ����ʼ�ձ���V1+V2=50mL�����ش��������⣺�о�С������ʵ��ʱ�����¶�

��1����С�ձ�����������ĭ���ϵ�����
���¡����ȣ���������ɢʧ
���¡����ȣ���������ɢʧ
����2��
����
����
����ܡ����ܡ��������β����������Ϊ����ͭ������ԭ�����������ȣ���������ɢʧ
�������ȣ���������ɢʧ
����3�����ձ����粻��Ӳֽ�壬������к�����ֵ��Ӱ����
ƫ��
ƫ��
���ƫ�ߡ���ƫ�͡�����Ӱ�족������4���������60mL 1.0mol?L-1�����50mL 1.1mol?L-1����������Һ���з�Ӧ����������ʵ����ȣ���������
����
����
�������ӡ��������١����䡱���������к�����ֵ����
����
�������ӡ��������١����䡱����5��ij�о�С�齫װ�����ƺ�V1 mL 1.0mol/L HCl��Һ��V2 mLδ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ��������¼��Һ�¶ȣ�ʵ������ͼ2��ʾ��ʵ����ʼ�ձ���V1+V2=50mL�����ش��������⣺�о�С������ʵ��ʱ�����¶�
����
����
������ڡ��������ڡ����ڡ���22�森��������1���������ȼƵĹ������жϸ�װ�õĴ�С�ձ�����������ĭ���ϵ����ã�
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�����
��3������Ӳֽ�壬����һ��������ɢʧ��
��4����Ӧ�ų����������������Լ�������Ķ����йأ��������к��ȵĸ����ʵ�����ش�
��5����ͼʾ�۲���ʼ�¶ȼ�Ϊʵ��ʱ�����¶ȣ�
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�����
��3������Ӳֽ�壬����һ��������ɢʧ��
��4����Ӧ�ų����������������Լ�������Ķ����йأ��������к��ȵĸ����ʵ�����ش�
��5����ͼʾ�۲���ʼ�¶ȼ�Ϊʵ��ʱ�����¶ȣ�
����⣺��1���������ȼƵĹ����ʵ��ijɰܹؼ����жϸ�װ�õĴ�С�ձ�����������ĭ���ϵ������DZ��¡����ȣ���������ɢʧ���ʴ�Ϊ�����¡����ȣ���������ɢʧ��
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹���������ͭ�ĵ���Ч�����ڻ��β�����������ʴ�Ϊ�����ܣ��������ȣ���������ɢʧ��
��3�����ձ����粻��Ӳֽ�壬����һ��������ɢʧ����õ��к�����ֵ�����С���ʴ�Ϊ��ƫ�ͣ�
��4����Ӧ�ų����������������Լ�������Ķ����йأ�������60mL0.25mol?L-1H2SO4��Һ��50mL0.55mol?L-1NaOH��Һ���з�Ӧ��������ʵ����ȣ�����ˮ�������࣬���ų�������ƫ�ߣ������к��ȵľ���ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ������������أ�������50mL0.50mol?L-1�������H2SO4��Һ��������ʵ�飬����к�����ֵ��ȣ��ʴ�Ϊ�����ӣ����䣻
��5������ʵ����ͼ2��ʾ���ݣ�����֪����ʵ�鿪ʼʱ�¶�һ���ǵ���22�棬�ʴ�Ϊ�����ڣ�
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹���������ͭ�ĵ���Ч�����ڻ��β�����������ʴ�Ϊ�����ܣ��������ȣ���������ɢʧ��
��3�����ձ����粻��Ӳֽ�壬����һ��������ɢʧ����õ��к�����ֵ�����С���ʴ�Ϊ��ƫ�ͣ�
��4����Ӧ�ų����������������Լ�������Ķ����йأ�������60mL0.25mol?L-1H2SO4��Һ��50mL0.55mol?L-1NaOH��Һ���з�Ӧ��������ʵ����ȣ�����ˮ�������࣬���ų�������ƫ�ߣ������к��ȵľ���ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ������������أ�������50mL0.50mol?L-1�������H2SO4��Һ��������ʵ�飬����к�����ֵ��ȣ��ʴ�Ϊ�����ӣ����䣻
��5������ʵ����ͼ2��ʾ���ݣ�����֪����ʵ�鿪ʼʱ�¶�һ���ǵ���22�棬�ʴ�Ϊ�����ڣ�
���������⿼��ѧ���й��к��ȵIJⶨ֪ʶ�����Ը�����ѧ֪ʶ���лش��ѶȲ���

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ijʵ��С�������50mL 1.0mol/L�����50mL 1.1mol/L����������Һ��ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�����ĭ���ϣ���ֽ������ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ���ϣ���ֽ���������ձ�������ĭ���ϰ壨��Ӳֽ�壩���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��Իش��������⣺
ijʵ��С�������50mL 1.0mol/L�����50mL 1.1mol/L����������Һ��ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�����ĭ���ϣ���ֽ������ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ���ϣ���ֽ���������ձ�������ĭ���ϰ壨��Ӳֽ�壩���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��Իش��������⣺ 50mL 1.0mol?L-1������50mL 1.1mol?L-1����������Һ����ͼװ���н����кͷ�Ӧ����ͨ���ⶨ��Ӧ���������ų��������������к��ȣ��Իش��������⣺
50mL 1.0mol?L-1������50mL 1.1mol?L-1����������Һ����ͼװ���н����кͷ�Ӧ����ͨ���ⶨ��Ӧ���������ų��������������к��ȣ��Իش��������⣺ 50mL 1.0mol?L-1�����50mL 1.1mol?L-1����������Һ��ͼװ���н����кͷ�Ӧ����ͨ���ⶨ��Ӧ���������ų��������������к��ȣ��Իش��������⣺
50mL 1.0mol?L-1�����50mL 1.1mol?L-1����������Һ��ͼװ���н����кͷ�Ӧ����ͨ���ⶨ��Ӧ���������ų��������������к��ȣ��Իش��������⣺