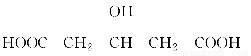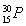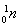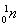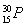جâؤ؟ؤعبف
ززثلززُ¥تاضطزھµؤسذ»ْ؛د³ةضذ¼نجه£¬¹م·؛س¦سأسع»¯ر§¹¤زµ،£تµرéتزضئ±¸ززثلززُ¥µؤ»¯ر§·½³جت½£؛CH3COOH£«C2H5OH CH3COOC2H5£«H2O£¬خھض¤أ÷إ¨ءٍثلشع¸أ·´س¦ضذئًµ½ءث´ك»¯¼ء؛حخüتص¼ءµؤ×÷سأ£¬ؤ³ح¬ر§ہûسأدآح¼ثùت¾×°ضأ½ّذذءثزشدآثؤ¸ِتµر飬تµرé؟ھت¼دبسأ¾ئ¾«µئخ¢بب3 min£¬شظ¼سببت¹ض®خ¢خ¢·ذجع3 min،£تµرé½لتّ؛َ³ن·ضصٌµ´تش¹ـ¢ٍشظ²âسذ»ْ²مµؤ؛ٌ¶ب£¬تµرé¼اآ¼بçدآ£؛
CH3COOC2H5£«H2O£¬خھض¤أ÷إ¨ءٍثلشع¸أ·´س¦ضذئًµ½ءث´ك»¯¼ء؛حخüتص¼ءµؤ×÷سأ£¬ؤ³ح¬ر§ہûسأدآح¼ثùت¾×°ضأ½ّذذءثزشدآثؤ¸ِتµر飬تµرé؟ھت¼دبسأ¾ئ¾«µئخ¢بب3 min£¬شظ¼سببت¹ض®خ¢خ¢·ذجع3 min،£تµرé½لتّ؛َ³ن·ضصٌµ´تش¹ـ¢ٍشظ²âسذ»ْ²مµؤ؛ٌ¶ب£¬تµرé¼اآ¼بçدآ£؛
تµرé±à؛إتش¹ـ¢ٌضذµؤتش¼ءتش¹ـ¢ٍضذتش¼ء²âµأسذ»ْ²مµؤ؛ٌ¶ب/cm
A2 mLزز´¼،¢2 mLززثل،¢1 mL 18 mol،¤L£1إ¨ءٍثل±¥؛حج¼ثلؤئبـز؛5.0
B3 mLزز´¼،¢2 mLززثل0.1
C3 mLزز´¼،¢2 mLززثل،¢6mL 3 mol،¤L£1ءٍثل1.2
D3 mLزز´¼،¢2 mLززثل،¢رخثل1.2
£¨1£©تµرéDµؤؤ؟µؤتاسëتµرéCدà¶شصص£¬ض¤أ÷H£«¶شُ¥»¯·´س¦¾كسذ´ك»¯×÷سأ،£تµرéDضذس¦¼سبëرخثلµؤجه»؛حإ¨¶ب·ض±ًتا________mL؛ح________mol/L،£
£¨2£©·ضخِتµرé________£¨جîتµرé±à؛إ£©µؤت¾ف£¬؟ةزشحئ²â³ِإ¨ءٍثلµؤخüث®ذشجل¸كءثززثلززُ¥µؤ²ْآت،£إ¨ءٍثلµؤخüث®ذشؤـ¹»جل¸كززثلززُ¥²ْآتµؤشزٍتا_______________________________________________________________،£
£¨3£©¼سببسذہûسعجل¸كززثلززُ¥µؤ²ْآت£¬µ«تµرé·¢دضخآ¶ب¹¸كززثلززُ¥µؤ²ْآت·´¶ّ½µµح£¬؟ةؤـµؤشزٍتا____________________________________________£¨´ً³ِء½جُ¼´؟ة£©،£
£¨1£©6،،6
£¨2£©A،¢C،،إ¨ءٍثل؟ةزشخüتصُ¥»¯·´س¦ةْ³ةµؤث®£¬ئ½؛âدٍةْ³ةززثلززُ¥µؤ·½دٍزئ¶¯
£¨3£©سذ´َء؟ززثل؛حزز´¼±»صô³ِ£»خآ¶ب¹¸ك¶ّ·¢ةْئنثû·´س¦
،¾½âخِ،؟£¨1£©H£«¶ش·´س¦µؤ´ك»¯×÷سأ£¬ذèزھب·±£c£¨H£«£©دàµب،¢بـز؛جه»دàµب،£