��Ŀ����
Ϊ�˲ⶨ![]() ��NaCl��KBr��ɵĻ�����и�������ռ��������������������ʵ�飺
��NaCl��KBr��ɵĻ�����и�������ռ��������������������ʵ�飺
(1)ȡ�����5g�������������ᣬ����![]() ����0.423L(��������
����0.423L(��������![]() ���ܶ�Ϊ1.964g/L)��
���ܶ�Ϊ1.964g/L)��
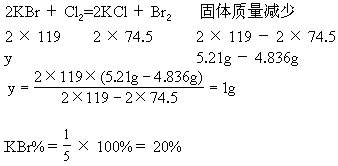
(2)����������Һ���������ù������������Ϊ5.21g��
(3)��(2)�еĹ�����������ˮ������![]() ��Ӧ���������ɺ�õ���������4.836g��
��Ӧ���������ɺ�õ���������4.836g��
�����������ݼ���ԭ�������![]() ��NaCl��KBr��ռ������������
��NaCl��KBr��ռ������������
�𰸣�
������
������
|
������(1)��ԭ������к�
(2)��ԭ�������KBr������Ϊy��
(3)NaCl%��100%��40%��20%��40% |

��ϰ��ϵ�д�
 ����ʦ���һ��һ��ϵ�д�
����ʦ���һ��һ��ϵ�д�
�����Ŀ
��9�֣�ijNaCl��Ʒ�л���NaBr���ʣ�Ϊ�˲ⶨ��NaCl��Ʒ�Ĵ��ȣ���Ƶ�ʵ���������ͼ��ʾ��

�Ķ�����ʵ����̣����������գ�

��1����ͼװ���������������Һ����ͨ�����Cl2��ʵ�飬�������������дװ�������ŵĻ�ѧҩƷ��
A | �� | B | �� | C | �� | D |
|
|
|
| ��Һ�� |
|
|
��2����ȡ����Ҫ����Ҫ����������_______________��
��3����Һ����ͨ��Cl2�Ļ�ѧ����ʽΪ ��
��4��ԭ������Ʒ���Ȼ��Ƶ���������Ϊ__________________��
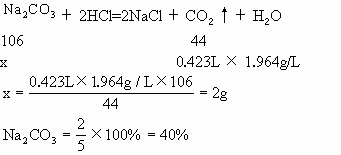
 ��ҵ�����Ĵ����г�����������NaCl���ʣ�ijУ�о���ѧϰ�С��Ϊ�˲ⶨ������д����������������ʹ����ͼʵ��װ�ã�˵�������Ӽ��ҵ���Ƥ�������п��ƣ����Ȳⶨһ��������Ʒ���ᷴӦ�ų�������̼���������ټ��������д��������������
��ҵ�����Ĵ����г�����������NaCl���ʣ�ijУ�о���ѧϰ�С��Ϊ�˲ⶨ������д����������������ʹ����ͼʵ��װ�ã�˵�������Ӽ��ҵ���Ƥ�������п��ƣ����Ȳⶨһ��������Ʒ���ᷴӦ�ų�������̼���������ټ��������д��������������