��Ŀ����
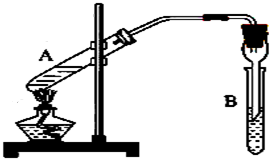
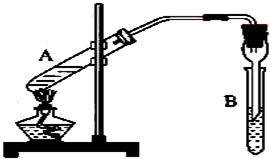
ʵ������ɲ���ͼװ����ȡ������������ش��������⣮��1���Թ�A��ʢ���Ҵ��������______��Ϊ�˵õ��ϴ��������������Թ�B������ʢ��______��
��2����Ӧʱ���Թ�A�ڳ��������������⣬����ˮ���ɣ���ˮ�����е���ԭ������______���ӣ�
��3����Ӧ�������Թ�B�ڵ���Һ��Ϊ���㣬��Ӧ���ɵ�����������______�㣨��д���ϡ����¡�����
��4��ʵ�������θ���ܵ����ó���ʹ����������������⣬������______��
��5���÷�Ӧ�Ļ�ѧ��Ӧ����ʽΪ��______��
���𰸡���������1���������Ҵ���Ũ���ᡢ���ȵ������·���������Ӧ��������������
�Ҵ������ᶼ�ӷ����Ʊ����������������Ҵ������ᣬͨ���ñ���̼������Һ���������������кͻӷ����������ᣬʹ֮ת��Ϊ����������ˮ�У�������������������ζ���ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�õ�����
��2�������봼������������Ӧ�У������е��Ȼ��ṩ-OH�����е�-OH�ṩ-H����������ˮ��
��3����������������ˮ���ܶȱ�ˮС��
��4��A�Թ����Ȳ��������θ���ܵĵ��ܿ�����Һ���¿��ܷ���������
��5���������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ���÷�ӦΪ���淴Ӧ��
����⣺��1���������Ҵ���Ũ���ᡢ���ȵ������·���������Ӧ��������������
�Ҵ������ᶼ�ӷ����Ʊ����������������Ҵ������ᣬͨ���ñ���̼������Һ���������������кͻӷ����������ᣬ�ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�õ�����
�ʴ�Ϊ��Ũ�������̼������Һ��
��2�������봼������������Ӧ�У������е��Ȼ��ṩ-OH�����е�-OH�ṩ-H����������ˮ����ˮ�е���ԭ���������
�ʴ�Ϊ�����
��3����������������ˮ����Һ�ֲ㣬�ܶȱ�ˮС�������������ϲ㣻
�ʴ�Ϊ���ϲ㣻
��4��A�Թ����Ȳ��������θ���ܵĹܿ�����Һ���¿��ܷ������������θ���ܵ����ó���ʹ����������������⣬�����Է�ֹ������
�ʴ�Ϊ����ֹ������
��5���������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ��ͬʱ�÷�Ӧ���棬��Ӧ�Ļ�ѧ����ʽΪ
CH3COOH+CH3CH2OH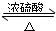 CH3COOC2H5+H2O��
CH3COOC2H5+H2O��
�ʴ�Ϊ��CH3COOH+HOCH2CH3 CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
���������⿼�������������Ʊ����ѶȲ���ע��ʵ����Һ�����ơ�����̼������Һ�������Լ�������Ӧ�Ļ�����
�Ҵ������ᶼ�ӷ����Ʊ����������������Ҵ������ᣬͨ���ñ���̼������Һ���������������кͻӷ����������ᣬʹ֮ת��Ϊ����������ˮ�У�������������������ζ���ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�õ�����
��2�������봼������������Ӧ�У������е��Ȼ��ṩ-OH�����е�-OH�ṩ-H����������ˮ��
��3����������������ˮ���ܶȱ�ˮС��
��4��A�Թ����Ȳ��������θ���ܵĵ��ܿ�����Һ���¿��ܷ���������
��5���������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ���÷�ӦΪ���淴Ӧ��
����⣺��1���������Ҵ���Ũ���ᡢ���ȵ������·���������Ӧ��������������
�Ҵ������ᶼ�ӷ����Ʊ����������������Ҵ������ᣬͨ���ñ���̼������Һ���������������кͻӷ����������ᣬ�ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�õ�����
�ʴ�Ϊ��Ũ�������̼������Һ��
��2�������봼������������Ӧ�У������е��Ȼ��ṩ-OH�����е�-OH�ṩ-H����������ˮ����ˮ�е���ԭ���������
�ʴ�Ϊ�����
��3����������������ˮ����Һ�ֲ㣬�ܶȱ�ˮС�������������ϲ㣻
�ʴ�Ϊ���ϲ㣻
��4��A�Թ����Ȳ��������θ���ܵĹܿ�����Һ���¿��ܷ������������θ���ܵ����ó���ʹ����������������⣬�����Է�ֹ������
�ʴ�Ϊ����ֹ������
��5���������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ��ͬʱ�÷�Ӧ���棬��Ӧ�Ļ�ѧ����ʽΪ
CH3COOH+CH3CH2OH
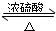 CH3COOC2H5+H2O��
CH3COOC2H5+H2O���ʴ�Ϊ��CH3COOH+HOCH2CH3
 CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O�����������⿼�������������Ʊ����ѶȲ���ע��ʵ����Һ�����ơ�����̼������Һ�������Լ�������Ӧ�Ļ�����

��ϰ��ϵ�д�
�����Ŀ

 ʵ������ɲ���ͼװ����ȡ������������ش��������⣮
ʵ������ɲ���ͼװ����ȡ������������ش��������⣮ CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O