��Ŀ����
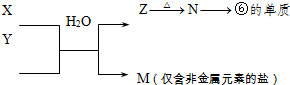
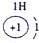
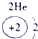
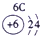
��12�֣��±�ΪԪ�����ڱ���һ���֣�����Ԫ�آ١����ڱ��е�λ�ã����û�ѧ����ش��������⣺
|
�� ���� |
IA |
|
0 |
|||||
|
1 |
�� |
��A |
��A |
��A |
��A |
��A |
��A |
|
|
2 |
|
|
|
|
|
�� |
�� |
|
|
3 |
�� |
|
�� |
|
|
�� |
�� |
|
��1���ܡ��ݡ��ߵ�ԭ�Ӱ뾶�ɴ�С��˳��Ϊ___________��
��2���͢ߵ���ۺ����������ǿ��Ϊ__ ___>_______��
��3���١�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬������ĵ���ʽΪ__________����������Һ�и������ܽ�Fe2+������д���÷�Ӧ�����ӷ���ʽ____________��
��4���ɱ���Ԫ���γɵ����ʿɷ�����ͼ�еķ�Ӧ������B��C��G�ǵ��ʣ�BΪ����ɫ���壬D��Һ�Լ��ԡ�

�� д��D��Һ��G��Ӧ�Ļ�ѧ����ʽ____________��
�� д������A��Һ�����ʵ������ӵķ���: ____________��
�� �����£������1 L 0.1 mol/L A��Һ��һ��ʱ�� ������ҺpHΪ12��������Һ����仯������õ�������ת�Ƶ��ӵ����ʵ���Ϊ__________ mol��
�� ����ͼ�и�����Ӧ��Ϊ��ȫת����������X�к��е�������_______��
��12�֣���1��Na > Cl > F ��1�֣� ��2��HClO4 > H2SO4 ��1�֣�
��3�� ��1�֣�
��1�֣�
H2O2 +2 Fe2����2H��= 2Fe3�� ��2H2O ��2�֣�
��4�� �� 2Al + 2NaOH + 2H2O = 2NaAlO2 +3H2�� ��2�֣�
��ȡ����A��Һ�μӼ���(ϡ�����ữ��)��������Һ�а�ɫ�������� ��1�֣�
��0.01 ��2�֣�
��Al (OH)3 H2O��NaCl ��2�֣�
��������

 �����ߴ���ϵ�д�
�����ߴ���ϵ�д�







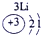
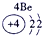
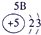
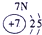
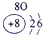
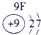
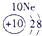
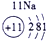





 ��ʾ����
��ʾ����