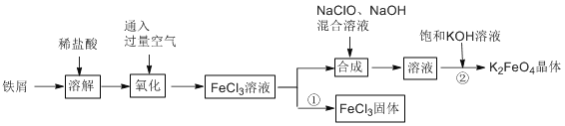
��Ŀ����
����Ŀ������������ȷ����
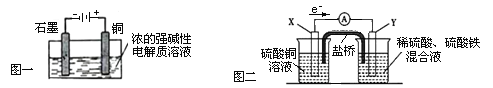
A��Cu2O��һ�ְ뵼����ϣ�������ɫ��ѧ������Ƶ���ȡCu2O�ĵ���ʾ��ͼ��ͼһ��ʾ��ʯī�缫�ϲ���������ͭ�缫����������Ӧ
B��ͼһ��ʾ����0.1mol����ת��ʱ����0.1molCu2O����
C��ͼ��װ���з�����Cu��2Fe3+ = Cu2+ ��2Fe2+ ��X���Ǹ�����Y�����Ͽ�����ͭ
D����ͼ�������ŵ������Ǵ��ݵ����ά�ֵ��ƽ�⣬Fe3+ �������Ž�������ձ���
���𰸡�A
��������
����ͼһΪ����,�������ҺΪǿ������Һ��ʯī�缫���Դ��������Ϊ������������ԭ��Ӧ��2H2O + 2e- = H2��+ 2OH-��ͭ�缫���Դ��������Ϊ����������������Ӧ��2Cu �C 2e- + 2OH- = Cu2O + H2O ������ܷ�ӦΪ��2Cu+H2OCu2O+H2�����ɴ˿�֪Aѡ����ȷ�����ݵ���ܷ�Ӧ��֪������0.2mol����ת��ʱ������0.1molCu2O���ɣ� Bѡ���ȷ��ͼ��װ��Ϊԭ��أ�����ͼ�и����ĵ����������ж�X���ǵ�صĸ�����Y���ǵ�ص�����������װ���з����ķ�Ӧ��Cu��2Fe3+ = Cu2+ ��2Fe2+����֪X��Ϊ������ʧ���ӣ�����������Ӧ����Cu-2e- = Cu2+������X���IJ���Ӧ����ͭ������ԭ��ص��γ�������Y������Ӧ���ǻ�Ա�ͭ���Ľ�����ʯī�Ȳ��ϣ���Cѡ���ȷ�����ŵ����������������ӵĶ���Ǩ�ƹ����˵���ͨ·���Ӷ���ͨ�ڵ�·���γɱպϻ�·�������˵���ͨ�����·�ӵ�ظ����������IJ���ת�ƣ�ʹԭ��ز��ϲ�����������ƽ���ɣ����ſ�ʹ�������ӵ����������е���Һ���ֵ����ԣ�ͬʱ������ֹ��Ӧ��ֱ�ӽӴ������ԣ�Fe3+ �Dz��ܾ������Ž�������ձ��У���Dѡ���ȷ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�