��Ŀ����
ʵ������ͼʾװ����ȡ����İ�����
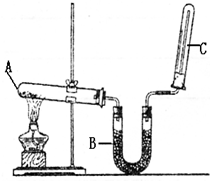
��1��д��A��������Ӧ�Ļ�ѧ����ʽ
��2��Bװ������ʢҩƷ������Ϊ
��3����μ��鰱���Ƿ��ռ�����

��1��д��A��������Ӧ�Ļ�ѧ����ʽ
Ca��OH��2+2 NH4 Cl�TCa Cl2 +2 H2O+2 NH3��
Ca��OH��2+2 NH4 Cl�TCa Cl2 +2 H2O+2 NH3��
����2��Bװ������ʢҩƷ������Ϊ
��ʯ��
��ʯ��
����3����μ��鰱���Ƿ��ռ�����
��ʪ��ĺ�ɫʯ����ֽ�����Թܿ��⣬���������ռ���
��ʪ��ĺ�ɫʯ����ֽ�����Թܿ��⣬���������ռ���
��������ʵ�������������ƺ��Ȼ���ڼ��������·�Ӧ�Ʊ�����������Ϊ�������壬�ü�ʯ�Ҹ�����鰱������ϸ��ĺ�ɫʯ����ֽ��ϸ���pH��ֽ�ȣ�
����⣺��1��ʵ�������������ƺ��Ȼ���ڼ��������·�Ӧ�Ʊ�������
��Ӧ�Ļ�ѧ����ʽΪCa��OH��2+2NH4Cl�TCaCl2 +2H2O+2 NH3�����ʴ�Ϊ��Ca��OH��2+2NH4Cl�TCaCl2 +2H2O+2 NH3����
��2��U�ι�ֻ��ʢװ��������������Ϊ�������壬�ü�ʯ�Ҹ���ʴ�Ϊ����ʯ�ң�
��3������Ϊ�������壬��ˮ��Ӧ����NH3?H2O�������ӳ�OH-���ӣ���Һ�ʼ��ԣ�����ʱ��ʪ��ĺ�ɫʯ����ֽ�����Թܿ��⣬���������ռ�����
�ʴ�Ϊ����ʪ��ĺ�ɫʯ����ֽ�����Թܿ��⣬���������ռ�����
��Ӧ�Ļ�ѧ����ʽΪCa��OH��2+2NH4Cl�TCaCl2 +2H2O+2 NH3�����ʴ�Ϊ��Ca��OH��2+2NH4Cl�TCaCl2 +2H2O+2 NH3����
��2��U�ι�ֻ��ʢװ��������������Ϊ�������壬�ü�ʯ�Ҹ���ʴ�Ϊ����ʯ�ң�
��3������Ϊ�������壬��ˮ��Ӧ����NH3?H2O�������ӳ�OH-���ӣ���Һ�ʼ��ԣ�����ʱ��ʪ��ĺ�ɫʯ����ֽ�����Թܿ��⣬���������ռ�����
�ʴ�Ϊ����ʪ��ĺ�ɫʯ����ֽ�����Թܿ��⣬���������ռ�����
���������⿼�鰱����ʵ�����Ʒ�����Ŀ�ѶȲ���ע�����֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
 �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�
�����Ŀ
 ʵ������ͼʾװ����ȡ����������
ʵ������ͼʾװ����ȡ����������
 ʵ������ͼʾװ����ȡ����������
ʵ������ͼʾװ����ȡ����������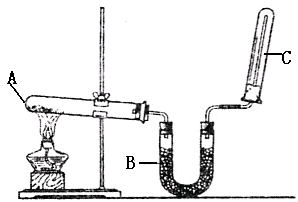 ʵ������ͼʾװ����ȡ����İ���
ʵ������ͼʾװ����ȡ����İ���