��Ŀ����
����Ŀ����һ�������£���ӦA(g)��B(g) C(g)��H < 0���ﵽƽ���������ͼ���жϣ�
��1�����£��ﵽ�µ�ƽ�������___________����
��2����ѹ���ﵽ�µ�ƽ�������______________����
��3������C������������ƽ�������_____________�� ��
��4������A������������ƽ�������____________������ʱB��ת����___________�������С�����䡱���¿�ͬ����
��5��ʹ�ô������ﵽƽ�������_______________����C����������_________ ��
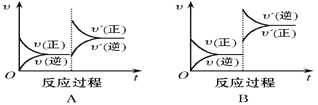
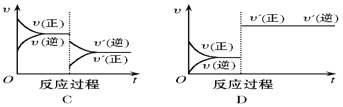
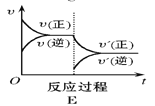
���𰸡�B C E A ���� D ����
��������
��ӦA(g)��B(g) C(g)��H < 0���ﵽƽ������¡�����Ӧ���Ũ�ȣ���Ӧ��������ѹ����С������Ũ�ȣ���Ӧ���ʼ�С������ƽ�������ƶ�����ѹƽ�������ƶ���ʹ�ô���ƽ�ⲻ�ƶ���
��ӦA(g)��B(g) C(g)��H < 0���ﵽƽ���
��1�����£����淴Ӧ���ʾ�����ƽ�������ƶ�����ﵽ�µ�ƽ���ͼΪB��
��2����ѹ�����淴Ӧ���ʾ���С��ƽ�������ƶ�����ﵽ�µ�ƽ���ͼΪC��
��3������C������˲���淴Ӧ���ʼ�С������Ӧ���ʲ��䣬���ŷ�Ӧ�Ľ��У�����Ӧ���������淴Ӧ���ʼ�С��������ƽ���ͼ��E��
��4������A������˲������Ӧ���������淴Ӧ���ʲ��䣬���ŷ�Ӧ�Ľ��У�����Ӧ���ʼ�С���淴Ӧ��������������ƽ���ͼΪA����ʱB��ת��������
��5��ʹ�ô��������淴Ӧ��������ƽ�ⲻ�ƶ�����ﵽƽ���ͼΪD��C�������������䡣

����Ŀ��A~H���ֶ���������Ԫ�������ڱ��е����λ����ͼ��ʾ����֪CԪ���γɵĵ�������������������������![]() ������ϼ�����ͻ��ϼ�֮��Ϊ2���ش��������⣺
������ϼ�����ͻ��ϼ�֮��Ϊ2���ش��������⣺
A | ���� | D | E | F | ||
B | C | ���� | G | H |
(1)DԪ�������ڱ��е�λ��Ϊ________________��
(2)����Ԫ�����γɵ���̬����������������ˮ��������________________(�ѧʽ)��
(3)B�ĵ�����F�ĵ�����ȼ�յĻ�����ɫΪ________��
(4)CԪ�صĵ��ʿ�����D��F�γɵ�һ����̬��������ȼ�գ�д���÷�Ӧ�Ļ�ѧ����ʽ_____








