��Ŀ����
��Ҫ��д��ѧ����ʽ����գ�
��1�������뱥����ˮ��Ӧ ��
��2���ۺ��� �䵥���� ��
�䵥���� ��
��3���Ҷ�����Ҷ��������۷�Ӧ ��
��4��HOCH2CH2COOH�����۷�Ӧ
��5���ױ��������ڹ��������µķ�Ӧ ��
��6�����������ڼ��������µķ�Ӧ ��
��7�������ǵĽṹ��ʽ
��8��CH2=C��CH3��COOCH3�ļӾ۷�Ӧ
��9��������̼��Ƶķ�Ӧ
��10���屽��ˮ�ⷴӦ
��11����������Һ�������̼�ķ�Ӧ
��12��2-�����������ķ�Ӧ ��
��1�������뱥����ˮ��Ӧ
��2���ۺ���
 �䵥����
�䵥������3���Ҷ�����Ҷ��������۷�Ӧ
��4��HOCH2CH2COOH�����۷�Ӧ
��5���ױ��������ڹ��������µķ�Ӧ
��6�����������ڼ��������µķ�Ӧ
��7�������ǵĽṹ��ʽ
��8��CH2=C��CH3��COOCH3�ļӾ۷�Ӧ
��9��������̼��Ƶķ�Ӧ
��10���屽��ˮ�ⷴӦ
��11����������Һ�������̼�ķ�Ӧ
��12��2-�����������ķ�Ӧ
���㣺��ѧ����ʽ����д
ר�⣺
��������1��������Ũ��ˮ��Ӧ���DZ��ӷ������ǻ��Ա���Ӱ�죬�ڶ�λ��ԭ�ӻ��ã�����ȡ����
��2�����ݾۺ���Ľṹ��֪���������������ǻ����Ȼ���ˮ�IJ��
��3���Ҷ�����Ҷ���֮�������ˮ�����γ���״�߾��
��4��HOCH2CH2COOH�������������۷�Ӧ�������ǻ������⣻
��5���ױ��������ڹ��������µķ�Ӧ������ȡ����
��6�����ڼ���������ˮ��ɿ�������C-O�����Ͽ���RCO-���ˮ�е�OH��-OR����ˮ�е�H��ˮ����������ʹ���
��7��������Ϊ���ǻ�ȩ������5���ǻ�һ��ȩ����
��8������ϩ����������Ӿ۷�Ӧ�õ��ۼ���ϩ�������
��9���������Ա�̼��ǿ����̼��Ƶķ�Ӧ��������ơ�ˮ��������̼��
��10���屽��NaOHˮ��Һ��ˮ�����ɱ��Ӻ�NaBr��
��11���������������̼��Ӧ���ɱ��Ӻ�̼�����ƣ�
��12��2-�������������ɱ�ͪ��
��2�����ݾۺ���Ľṹ��֪���������������ǻ����Ȼ���ˮ�IJ��
��3���Ҷ�����Ҷ���֮�������ˮ�����γ���״�߾��
��4��HOCH2CH2COOH�������������۷�Ӧ�������ǻ������⣻
��5���ױ��������ڹ��������µķ�Ӧ������ȡ����
��6�����ڼ���������ˮ��ɿ�������C-O�����Ͽ���RCO-���ˮ�е�OH��-OR����ˮ�е�H��ˮ����������ʹ���
��7��������Ϊ���ǻ�ȩ������5���ǻ�һ��ȩ����
��8������ϩ����������Ӿ۷�Ӧ�õ��ۼ���ϩ�������
��9���������Ա�̼��ǿ����̼��Ƶķ�Ӧ��������ơ�ˮ��������̼��
��10���屽��NaOHˮ��Һ��ˮ�����ɱ��Ӻ�NaBr��
��11���������������̼��Ӧ���ɱ��Ӻ�̼�����ƣ�
��12��2-�������������ɱ�ͪ��
���
�⣺��1��������Ũ��ˮ��Ӧ���DZ��ӷ������ǻ��Ա���Ӱ�죬�ڶ�λ��ԭ�ӻ��ã�����ȡ����Ӧ�Ļ�ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��2�����ݾۺ���Ľṹ��֪���������������ǻ����Ȼ���ˮ�IJ���䵥��Ϊ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��3���Ҷ�����Ҷ���֮�������ˮ�����γ���״�߾����Ӧ�Ļ�ѧ����ʽΪnHOCH2CH2OH+nHOOC-COOH
 +��2n-1��H2O��
+��2n-1��H2O��
�ʴ�Ϊ��nHOCH2CH2OH+nHOOC-COOH
 +��2n-1��H2O��
+��2n-1��H2O��
��4��HOCH2CH2COOH�������۷�Ӧ��nHOCH2CH2COOH
 +��n-1��H2O��
+��n-1��H2O��
�ʴ�Ϊ��nHOCH2CH2COOH
 +��n-1��H2O��
+��n-1��H2O��
��5���ױ��������ڹ����������ܷ���ȡ����Ӧ����ʱֻ��ȡ�����ϵ���ԭ�ӣ�����ȡ�������ϵ���ԭ�ӣ���ԭ�ӵ�ȡ���Ƿֲ�ͬʱ���еģ�һԪȡ����ֻȡ�����ϵ�һ����ԭ�ӣ�C6H5CH3+Cl2
C6H5CH2Cl+HCl��
�ʴ�Ϊ��C6H5CH3+Cl2
C6H5CH2Cl+HCl��
��6�����������ڼ��������µķ�ӦHCOOC2H5+NaOH
HCOONa+C2H5OH��
�ʴ�Ϊ��HCOOC2H5+NaOH
HCOONa+C2H5OH��
��7�������ǽṹ��ʽΪ��CH2OHCHOHCHOHCHOHCHOHCHO��
�ʴ�Ϊ��CH2OHCHOHCHOHCHOHCHOHCHO��
��8������ϩ����������Ӿ۷�ӦΪ��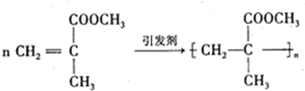 ��
��
�ʴ�Ϊ�� ��
��
��9��������̼��Ƶķ�Ӧ2CH3COOH+CaCO3=��CH3COO��2Ca+H2O+CO2����
�ʴ�Ϊ��2CH3COOH+CaCO3=��CH3COO��2Ca+H2O+CO2����
��10���屽��NaOHˮ��Һ��ˮ�����ɱ��Ӻ�NaBr����ӦΪC6H5Br+NaOH
C6H5OH+NaBr��
�ʴ�Ϊ��C6H5Br+NaOH
C6H5OH+NaBr��
��11���������������̼��Ӧ���ɱ��Ӻ�̼�����ƣ���Ӧ�Ļ�ѧ����ʽΪ��C6H5ONa+CO2+H2O��C6H5OH+NaHCO3��
�ʴ�Ϊ��C6H5ONa+CO2+H2O��C6H5OH+NaHCO3��
��12��2-�����Ĵ��������ɱ�ͪ��ˮ����Ӧ�ķ���ʽΪ��2CH3CHOHCH3+O2
2CH3COCH3+2H2O��
�ʴ�Ϊ��2CH3CHOHCH3+O2
2CH3COCH3+2H2O��
 ��
���ʴ�Ϊ��
 ��
����2�����ݾۺ���Ľṹ��֪���������������ǻ����Ȼ���ˮ�IJ���䵥��Ϊ��
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����3���Ҷ�����Ҷ���֮�������ˮ�����γ���״�߾����Ӧ�Ļ�ѧ����ʽΪnHOCH2CH2OH+nHOOC-COOH
| Ũ���� |
| �� |
 +��2n-1��H2O��
+��2n-1��H2O���ʴ�Ϊ��nHOCH2CH2OH+nHOOC-COOH
| Ũ���� |
| �� |
 +��2n-1��H2O��
+��2n-1��H2O����4��HOCH2CH2COOH�������۷�Ӧ��nHOCH2CH2COOH
| ���� |
| �� |
 +��n-1��H2O��
+��n-1��H2O���ʴ�Ϊ��nHOCH2CH2COOH
| ���� |
| �� |
 +��n-1��H2O��
+��n-1��H2O����5���ױ��������ڹ����������ܷ���ȡ����Ӧ����ʱֻ��ȡ�����ϵ���ԭ�ӣ�����ȡ�������ϵ���ԭ�ӣ���ԭ�ӵ�ȡ���Ƿֲ�ͬʱ���еģ�һԪȡ����ֻȡ�����ϵ�һ����ԭ�ӣ�C6H5CH3+Cl2
| ���� |
�ʴ�Ϊ��C6H5CH3+Cl2
| ���� |
��6�����������ڼ��������µķ�ӦHCOOC2H5+NaOH
| �� |
�ʴ�Ϊ��HCOOC2H5+NaOH
| �� |
��7�������ǽṹ��ʽΪ��CH2OHCHOHCHOHCHOHCHOHCHO��
�ʴ�Ϊ��CH2OHCHOHCHOHCHOHCHOHCHO��
��8������ϩ����������Ӿ۷�ӦΪ��
 ��
���ʴ�Ϊ��
 ��
����9��������̼��Ƶķ�Ӧ2CH3COOH+CaCO3=��CH3COO��2Ca+H2O+CO2����
�ʴ�Ϊ��2CH3COOH+CaCO3=��CH3COO��2Ca+H2O+CO2����
��10���屽��NaOHˮ��Һ��ˮ�����ɱ��Ӻ�NaBr����ӦΪC6H5Br+NaOH
| ˮ |
| �� |
�ʴ�Ϊ��C6H5Br+NaOH
| ˮ |
| �� |
��11���������������̼��Ӧ���ɱ��Ӻ�̼�����ƣ���Ӧ�Ļ�ѧ����ʽΪ��C6H5ONa+CO2+H2O��C6H5OH+NaHCO3��
�ʴ�Ϊ��C6H5ONa+CO2+H2O��C6H5OH+NaHCO3��
��12��2-�����Ĵ��������ɱ�ͪ��ˮ����Ӧ�ķ���ʽΪ��2CH3CHOHCH3+O2
| ͭ���� |
| �� |
�ʴ�Ϊ��2CH3CHOHCH3+O2
| ͭ���� |
| �� |
���������⿼���˻�ѧ����ʽ����д����Ŀ�Ѷ��еȣ��ڽ�����ʽ����д��ʱ������ȷ����Ӧԭ����Ȼ��������ԭ���ҳ���Ӧ�������ͷ�Ӧ���������ݷ���ʽ����д������д����ʽ��

��ϰ��ϵ�д�
�����Ŀ
�Է�Ӧ2Al+2NaOH+2H2O�T2NaAlO2+3H2����˵����ȷ���ǣ�������
| A����������NaOH |
| B������3mol H2ʱת����6mol���� |
| C����Ӧ�е�Al����ԭ |
| D��1molAlʧ���ӣ���3molH2O�е���õ��� |
ijС����е绯ѧ�о�����ͬѧ�������ͼ��װ��ͼ����ͬѧ���ü�װ�ú�����������ҩƷ����ϣ�������ɵ�ʵ���ǣ�������


| A��ʹ��ͬѧ��������ɸ��� |
| B�����һ���µ�ԭ��� |
| C����̼�缫�϶�п |
| D��ʹп�缫�ܵ����� |
������0.5molNa2SO4��ˮ��Һ�У����е�Na+�������ǣ�����������
| A��3.01��1023 |
| B��6.02��1023 |
| C��0.5 |
| D��1 |
���л�ѧ��Ӧ�У�����������ԭ��Ӧ���ǣ�������
| A��Cu+4 HNO3��Ũ���TCu��NO3��2+2 NO2��+2 H2O |
| B��CuO+2HCl�TCuCl2+H2O |
| C��HCl+NaHCO3=NaCl+H2O+CO2 |
| D��CuCl2+2NaOH=Cu��OH��2��+2NaCl |