��Ŀ����
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ�-���ڱ��е�λ�ã��ش���������

��1���ݢޢ������Ӱ뾶�ɴ�С��˳��Ϊ______________�������ӷ��ű�ʾ����
��2���ȽϢܢ���⻯��е�ߵ�˳��˵������____________________________��
��3��lg���ʢ��ڳ�������ȫȼ��������̬����ų�QkJ����������д�����ʾȼ�յ��Ȼ�ѧ����ʽ
______________________________��
��4���ɱ�������Ԫ����ɵĵ����������ͬ�Ļ�����Ļ�ѧʽΪ________________��
��5���ɢߢ�����Ԫ����ɵ�һ�ֻ������ˮ��Һ��_________�ԣ���ᡱ��������С�������ԭ��_____________________�������ӷ���ʽ�����
��6���ɢܢ�����Ԫ����ɵ�һ�ֻ�������뵽����������Һ�У��������ɫ���������������������д���÷�Ӧ�����ӷ���ʽ_____________________________��
��2���ȽϢܢ���⻯��е�ߵ�˳��˵������____________________________��
��3��lg���ʢ��ڳ�������ȫȼ��������̬����ų�QkJ����������д�����ʾȼ�յ��Ȼ�ѧ����ʽ
______________________________��
��4���ɱ�������Ԫ����ɵĵ����������ͬ�Ļ�����Ļ�ѧʽΪ________________��
��5���ɢߢ�����Ԫ����ɵ�һ�ֻ������ˮ��Һ��_________�ԣ���ᡱ��������С�������ԭ��_____________________�������ӷ���ʽ�����
��6���ɢܢ�����Ԫ����ɵ�һ�ֻ�������뵽����������Һ�У��������ɫ���������������������д���÷�Ӧ�����ӷ���ʽ_____________________________��
��1��S2->Cl->Na+>Mg2+
��2��H2O�ķе����H2S��ԭ����Һ̬ˮ���Ӽ�������
��3��H2(g)+1/2O2(g)==H2O(g) ��H= -2QkJ/mol����2H2(g)+O2(g)==2H2O(g) ��H=-4QkJ/mol��
��4��C2H4
��5���Al3++3H2O Al(OH)3+3H+
Al(OH)3+3H+
��6��3Na2O2+6Fe2++6H2O==6Na++4Fe(OH)3��+2Fe3+
��2��H2O�ķе����H2S��ԭ����Һ̬ˮ���Ӽ�������
��3��H2(g)+1/2O2(g)==H2O(g) ��H= -2QkJ/mol����2H2(g)+O2(g)==2H2O(g) ��H=-4QkJ/mol��
��4��C2H4
��5���Al3++3H2O
 Al(OH)3+3H+
Al(OH)3+3H+ ��6��3Na2O2+6Fe2++6H2O==6Na++4Fe(OH)3��+2Fe3+

��ϰ��ϵ�д�
�����Ŀ








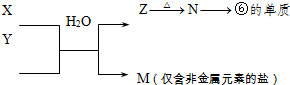
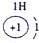
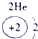
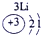
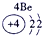
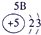
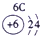
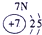
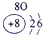
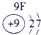
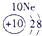
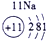





 ��ʾ����
��ʾ����