��Ŀ����
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ٣����ڱ��е�λ�ã��û�ѧ����ش��������⣺
| �� ���� | IA | | 0 | |||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | �� | | �� | �� | �� | |
��1��Ԫ�آ�������������������ȵ�ԭ�ӷ�����________________��
��2���ܡ�������Ԫ��ԭ�Ӱ�1��1��ɵij���������Ļ�ѧʽ________________��
��3���ۡ���͢������������Ӧˮ�����������ǿ������˳����________________��
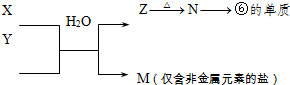
��4���õ���ʽ��ʾ�ٺ͢��γɻ�����Ĺ���_________________________��
���ɱ���Ԫ���γɵ�����A��B��C�ɷ������·�Ӧ(����������ȥ)���Իش�

��1����X��ǿ�����Ե��ʣ���A��������______________
a��S b��N2 c��Na d��Mg e��Al
��2����X��һ�ֳ������ɽ������ʣ���C��ˮ��Һ�еμ���������Һ������������ϡ����İ�ɫ�����������C��Һ�н������ӵķ�����____________________________________
__________________________����֪��������Һ�иý��������ܱ�˫��ˮ������д���÷�Ӧ�����ӷ���ʽ��___________________________________________��
��12�֣���1��3216S (2) Na2O2��3��HClO4��HNO3��H3PO4
(4)
��1��de ��2��ȡ������Һ���Թ��У���������������Һ��������ɫ�����漴��ɻ���ɫ����ɺ��ɫ�������ȼ���KSCN��Һ������ͨ���������Ѫ��ɫ�������������𰸾��ɣ���
2Fe ��H2O2��2H����2Fe
��H2O2��2H����2Fe ��2H2O����1����2��1������ÿС��2�֣�
��2H2O����1����2��1������ÿС��2�֣�
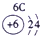
�����������������Ԫ�������ڱ��е�λ�ÿ�֪���١���ֱ���H��C��N��O��Na��Al��S��Cl��P���ݴ˿�֪��
��1��Ԫ�آ�������������������ȵ�ԭ�ӷ����� ��
��
��2���ܡ�������Ԫ��ԭ�Ӱ�1��1��ɵij���������Ļ�ѧʽNa2O2��
��3���ǽ�����Խǿ������������ˮ���������Խǿ����ۡ���͢������������Ӧˮ�����������ǿ������˳����HClO4��HNO3��H3PO4��
��4���ٺ͢��γɻ�����NaH���������Ӽ������ӻ���������õ���ʽ��ʾ�ٺ͢��γɻ�����Ĺ����� ��
��
��1����X��ǿ�����Ե��ʣ���A������̼��S�������ˮ�Ƶȣ�����������þ��������ѡde��
��2����X��һ�ֳ������ɽ������ʣ����X��������C��ˮ��Һ�еμ���������Һ������������ϡ����İ�ɫ��������˵��C���Ȼ���������A��������B���Ȼ����������������ӵķ�����ȡ������Һ���Թ��У���������������Һ��������ɫ�����漴��ɻ���ɫ����ɺ��ɫ�������ȼ���KSCN��Һ������ͨ���������Ѫ��ɫ�������������𰸾��ɣ���˫��ˮ�Ļ�ԭ������ˮ�����Է�Ӧ�����ӷ���ʽ��2Fe ��H2O2��2H����2Fe
��H2O2��2H����2Fe ��2H2O��
��2H2O��
���㣺����Ԫ�����ڱ��Ľṹ�Լ�Ԫ�������ɵ�Ӧ�ú��жϡ�Ԫ�ؼ��仯����ת�����й��ж�
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ�����ض�ѧ������֪ʶ�Ĺ��̺�ѵ�������������ѧ����������������Ӧ��������������Ҫ��Ԫ�ء�λ�������ԡ����߹�ϵ���ۺϿ��飬�Ƚ�ȫ�濼��ѧ���й�Ԫ���ƶ�֪ʶ���������֪ʶ�������������ԡ����ڱ���Ԫ�ص��ƶϡ�Ϊ���壬����ѧ����Ԫ�����ڱ�����Ϥ�̶ȼ���Ա��и�Ԫ�����ʺ���Ӧԭ�ӽṹ�������Եݱ���ɵ���ʶ�����ճ̶ȡ�������ѧ�������ʽṹ�����ʹ�ϵ�Լ�����Ԫ�������ɽ�����廯ѧ�����������

 ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д� �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д� ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д� һ���㶨ϵ�д�
һ���㶨ϵ�д� ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д�







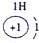
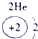
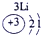
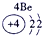
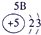
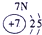
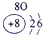
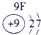
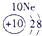
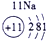





 ��ʾ����
��ʾ����