��Ŀ����
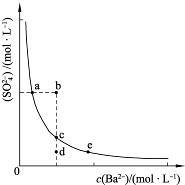
SO2�����Ṥҵβ������Ҫ�ɷ֣�ʵ�����У�������ͼ��ʾ���̣��ⶨ��״���£����ΪV L�����Ṥҵβ����SO2�ĺ�����
��1��������м���H2O2��Һʱ������Ӧ�����ӷ���ʽΪ______��1mol H2O2�μӷ�Ӧ��ת�Ƶĵ�����Ϊ______��
��2������۵IJ��������ǣ�����______��______��______�����أ�
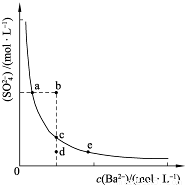
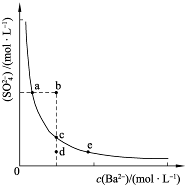
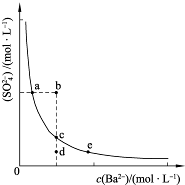
��3��һ���¶��£�BaSO4�ij����ܽ�ƽ��������ͼ��ʾ���������μ���Ba��OH��2��Һ�Ĺ����У�BaSO4���ܶȻ�����______�����������С���������䡱֮һ������Һ��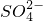 Ũ�ȵı仯���Ϊ______������ţ�
Ũ�ȵı仯���Ϊ______������ţ�
��d��c��e�� ��b��c��d�� ��a��c��e�� ��d��c��a
��4����V Lβ����SO2���������Ϊ______���ú���V��m�Ĵ���ʽ��ʾ����
�⣺��1��������м���H2O2��Һ������������������ԣ�����������л�ԭ�Ա�����Ϊ���ᣬ������Ӧ�����ӷ���ʽΪH2O2+SO2=2H+++SO42-��1mol�������ⷴӦת�Ƶ������ʵ���Ϊ2mol��ת�Ƶĵ�����Ϊ2��6.02��1023=1.204��1024��
�ʴ�Ϊ��H2O2+SO2=2H+++SO42-��1.204��1024��
��2������۵IJ����Ǵ���Һ�з�����������ᱵ�����������ǹ��ˡ�ϴ�ӡ�������أ�
�ʴ�Ϊ��ϴ�ӣ����
��3�������ܽ�ƽ�������������Ũ�Ⱥͱ�����Ũ�ȳ˻�Ϊ���������ż���ı�����Ũ���������������Ũ�ȼ�С��ʼ���DZ�����Һ�еij����ܽ�ƽ�⣬Ӧ�������ϱ仯��bd�㲻�Ǹ��¶��µı�����Һ��
�ʴ�Ϊ�����䣬�ۣ�
��4��mg�����ᱵ�����������ᱵ�����ʵ���Ϊ =
= mol��������Ԫ���غ��֪������������Ϊ
mol��������Ԫ���غ��֪������������Ϊ mol��22.4L/mol=
mol��22.4L/mol= L����β���ж���������������=
L����β���ж���������������= =
= ��100%��
��100%��
�ʴ𰸣� ��100%��
��100%��
��������1�������з����ķ�ӦΪ��SO2+H2O2=H2SO4��H2SO4+Ba��OH��2=BaSO4��+2H2O��
��2������۵IJ����Ǵ���Һ�з�����������ᱵ�����������ǹ��ˡ�ϴ�ӡ�������أ�
��3���ܶȻ��������¶ȱ仯������Ũ�ȸı䣻�����ܽ�ƽ�������������Ũ�Ⱥͱ�����Ũ�ȳ˻�Ϊ���������ż���ı�����Ũ���������������Ũ�ȼ�С��ʼ���DZ�����Һ�еij����ܽ�ƽ�⣬Ӧ�������ϱ仯��
��4��mg�����ᱵ���������������ᱵ�����ʵ�����������Ԫ���غ������������������������������������������
���������⿼��ѧ����ʵ��ԭ����ʵ����������⡢ʵ�鷽����ơ�Ԫ�ػ��������ʡ���ѧ���㡢�����ܽ�ƽ��ȣ��Ѷ��еȣ����ʵ��ԭ���ǽ���Ĺؼ�����Ҫѧ���߱���ʵ�Ļ���֪ʶ���ۺ�����֪ʶ�������⡢��������������
�ʴ�Ϊ��H2O2+SO2=2H+++SO42-��1.204��1024��
��2������۵IJ����Ǵ���Һ�з�����������ᱵ�����������ǹ��ˡ�ϴ�ӡ�������أ�
�ʴ�Ϊ��ϴ�ӣ����
��3�������ܽ�ƽ�������������Ũ�Ⱥͱ�����Ũ�ȳ˻�Ϊ���������ż���ı�����Ũ���������������Ũ�ȼ�С��ʼ���DZ�����Һ�еij����ܽ�ƽ�⣬Ӧ�������ϱ仯��bd�㲻�Ǹ��¶��µı�����Һ��
�ʴ�Ϊ�����䣬�ۣ�
��4��mg�����ᱵ�����������ᱵ�����ʵ���Ϊ
 =
= mol��������Ԫ���غ��֪������������Ϊ
mol��������Ԫ���غ��֪������������Ϊ mol��22.4L/mol=
mol��22.4L/mol= L����β���ж���������������=
L����β���ж���������������= =
= ��100%��
��100%���ʴ𰸣�
 ��100%��
��100%����������1�������з����ķ�ӦΪ��SO2+H2O2=H2SO4��H2SO4+Ba��OH��2=BaSO4��+2H2O��
��2������۵IJ����Ǵ���Һ�з�����������ᱵ�����������ǹ��ˡ�ϴ�ӡ�������أ�
��3���ܶȻ��������¶ȱ仯������Ũ�ȸı䣻�����ܽ�ƽ�������������Ũ�Ⱥͱ�����Ũ�ȳ˻�Ϊ���������ż���ı�����Ũ���������������Ũ�ȼ�С��ʼ���DZ�����Һ�еij����ܽ�ƽ�⣬Ӧ�������ϱ仯��
��4��mg�����ᱵ���������������ᱵ�����ʵ�����������Ԫ���غ������������������������������������������
���������⿼��ѧ����ʵ��ԭ����ʵ����������⡢ʵ�鷽����ơ�Ԫ�ػ��������ʡ���ѧ���㡢�����ܽ�ƽ��ȣ��Ѷ��еȣ����ʵ��ԭ���ǽ���Ĺؼ�����Ҫѧ���߱���ʵ�Ļ���֪ʶ���ۺ�����֪ʶ�������⡢��������������

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
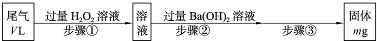


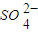 Ũ�ȵı仯���Ϊ______������ţ�
Ũ�ȵı仯���Ϊ______������ţ�