��Ŀ����
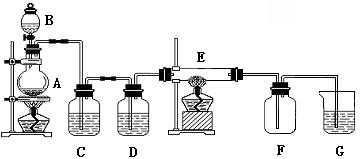
����ͼ��ʾ��װ���У�A����������װ�ã�C��D Ϊ���徻��װ�ã�C��װ�б���ʳ��ˮ��D ��װ��Ũ���ᣩ��E ��Ӳ�ʲ�����װ��ϸ��˿����FΪ����Ŀչ��ƿ���ձ�G ��װ������������Һ��
�Իش�
��1��ʵ�����������Ļ�ѧ����ʽ��_______________________________��
��2��Cװ�õ�������________________��D װ�õ�������__________________��
E�з�����ѧ��Ӧ�ķ���ʽΪ��_____________________________��
��3���ձ�G ��װ������������Һ��������___________________��������Ӧ�Ļ�ѧ����ʽΪ��_______________________________________��
��1��MnO2 + 4 HCl(Ũ) = MnCl2 + Cl2��+2 H2O
��2����ȥ�����л��е��Ȼ��� ��������
2 Fe + 3 Cl2= 2 FeCl3
��3�����ն��������
Cl2 + 2 NaOH= NaCl + NaClO + H2O
����:

��ϰ��ϵ�д�
�����Ŀ