��Ŀ����
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ�-���ڱ��е�λ�ã��û�ѧ����ش��������⣺
��1���ܡ��ݡ���ԭ�Ӱ뾶�ɴ�С��˳��Ϊ______��
��2���ڡ��ۡ��ߵ���ۺ������������ǿ������˳����______��
��3���١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ��______��
��4���ĵ�����ݵ�����������ˮ�������Ӧ�Ļ�ѧ����ʽ��______��
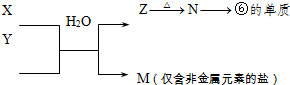
��5���ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ______��
| �� ���� |
IA | 0 | ||||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | ||||
��2���ڡ��ۡ��ߵ���ۺ������������ǿ������˳����______��
��3���١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ��______��
��4���ĵ�����ݵ�����������ˮ�������Ӧ�Ļ�ѧ����ʽ��______��
��5���ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ______��

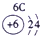
����Ԫ�������ڱ��еķֲ�������֪������H������C������N������O������Na������Al������Si������Cl
��1�����Ӳ�Խ��뾶Խ������Na��Al��O�����Ӳ�һ�����ԭ�ӣ��˵����Խ��뾶ԽС����Na��Al���ʴ�Ϊ��Na��Al��O��
��2���ڢ���C��N����ͬһ����Ԫ�ص�ԭ�ӣ�����Ԫ�������ɣ�����������������Ӧˮ�������������ǿ���������ԣ�HNO3��H2CO3���ڢ���C��Si����ͬ����Ԫ�أ�����Ԫ�������ɣ����ϵ�������������Ӧˮ���������������H2CO3��H2SiO3�ʴ�Ϊ��HNO3��H2CO3��H2SiO3��
��3���٢ܢݢ�ֱ���H��O��Na��Cl��������Ԫ�ء���ԭ�ӡ���Ԫ����ɵ��������ƣ���Ԫ�ء���ԭ�ӡ���Ԫ����ɵĴ������ƣ����ǼȺ����Ӽ��ֺ����Թ��ۼ��Ļ��������ʽΪ����Na+

��
�ʴ�Ϊ��Na+

��
��4���ĵ���Al���Na������������ˮ����NaOH������Ӧ����ƫ�����ƺ���������ѧ����ʽΪ��2Al+2NaOH+2H2O=2NaOH+3H2�����ʴ�Ϊ��2Al+2NaOH+2H2O=2NaOH+3H2����
��5���ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ�����ͼת���ķ�Ӧ������M�ǽ����ǽ������Σ�����һ������Σ�Z
N���ĵ��ʣ��������ڱ��Ľṹ�жϢ��ǽ������������ƶ�N��������������ɽ�������Z�������������ȷֽ����������������������Ϸ�Ӧ��X+Y+H2O��Al��OH��3+NH4+ ��֪XY�����κ�һˮ�ϰ��ķ�Ӧ������X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ��
Al3++3NH3+3H2O=Al��OH��3��+3NH4+���ʴ�Ϊ��Al3++3NH3+3H2O=Al��OH��3��+3NH4+��
��1�����Ӳ�Խ��뾶Խ������Na��Al��O�����Ӳ�һ�����ԭ�ӣ��˵����Խ��뾶ԽС����Na��Al���ʴ�Ϊ��Na��Al��O��
��2���ڢ���C��N����ͬһ����Ԫ�ص�ԭ�ӣ�����Ԫ�������ɣ�����������������Ӧˮ�������������ǿ���������ԣ�HNO3��H2CO3���ڢ���C��Si����ͬ����Ԫ�أ�����Ԫ�������ɣ����ϵ�������������Ӧˮ���������������H2CO3��H2SiO3�ʴ�Ϊ��HNO3��H2CO3��H2SiO3��
��3���٢ܢݢ�ֱ���H��O��Na��Cl��������Ԫ�ء���ԭ�ӡ���Ԫ����ɵ��������ƣ���Ԫ�ء���ԭ�ӡ���Ԫ����ɵĴ������ƣ����ǼȺ����Ӽ��ֺ����Թ��ۼ��Ļ��������ʽΪ����Na+

��
�ʴ�Ϊ��Na+

��
��4���ĵ���Al���Na������������ˮ����NaOH������Ӧ����ƫ�����ƺ���������ѧ����ʽΪ��2Al+2NaOH+2H2O=2NaOH+3H2�����ʴ�Ϊ��2Al+2NaOH+2H2O=2NaOH+3H2����
��5���ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ�����ͼת���ķ�Ӧ������M�ǽ����ǽ������Σ�����һ������Σ�Z
| ���� |
Al3++3NH3+3H2O=Al��OH��3��+3NH4+���ʴ�Ϊ��Al3++3NH3+3H2O=Al��OH��3��+3NH4+��

��ϰ��ϵ�д�
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
�����Ŀ








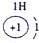
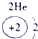
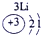
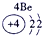
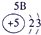
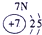
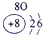
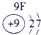
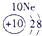
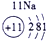





 ��ʾ����
��ʾ����