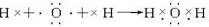��Ŀ����
�ش��������⣺
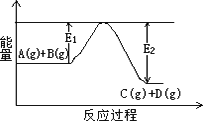
(1)��ӦA(g)��B(g)![]() C(g)��D(g)�����е������仯��ͼ��ʾ���жϸ÷�Ӧ��H________0(�������������������ȷ����)��
C(g)��D(g)�����е������仯��ͼ��ʾ���жϸ÷�Ӧ��H________0(�������������������ȷ����)��
(2)��Al2O3��Ni������̬���ᷢ�����з�Ӧ��
����(g)��CO(g)��H2O(g)����H1����34.0 kJ/mol
����(g)��CO2(g)��H2(g)����H2����7.0 kJ/mol
�����ķ���ʽΪ________���ڸ������£���̬CO2����̬H2������̬CO����̬H2O���Ȼ�ѧ����ʽΪ________��
(3)����ƽ�����ʢ��ǿ��ԭ��Һ̬��(N2H4)��ǿ������Һ̬˫��ˮ(H2O2)��
����0.4 molҺ̬�º�0.8 molҺ̬H2O2��Ϸ�Ӧ�����ɵ�����ˮ�������ų�256.7 kJ������(�൱��25�桢101 kPa�²�õ�����)����Ӧ���Ȼ�ѧ����ʽΪ________��
�𰸣�
������
������
����(1)��(1��)
����(2)CH2O2(1��)��CO2(g)��H2(g)��CO(g)��H2O(g)����H����41.0 kJ��mol��1(2��)
����(3)N2H4(l)��2H2O2(l)��N2(g)��4H2O(g)��
��H����641.75 kJ��mol��1(2��)
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ